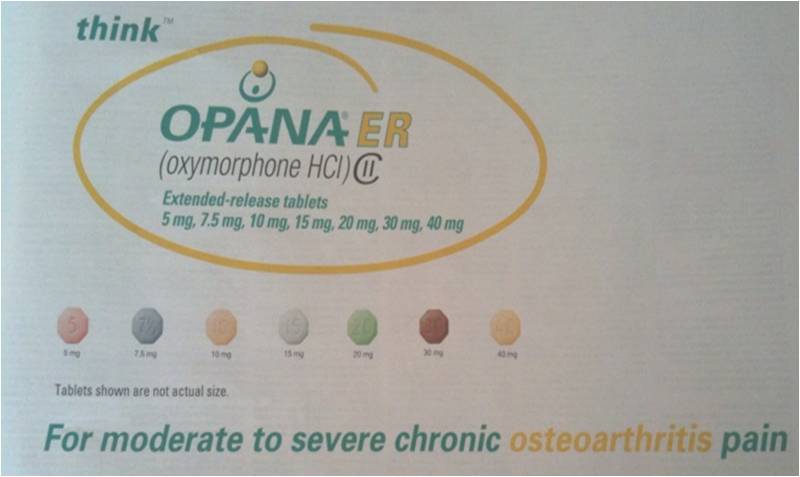
A High Dose Narcotic for Moderate Knee Arthritis??
In the truth is stranger than fiction category, I came across the above ad this morning in a pain management throwaway journal mailed to my house. For the non-medical types reading this post, Opna ER is a crazy strong narcotic usually used in end of life pain or for the most severe and intractable chronic pain patients (the guy with 6 failed back surgeries and severe nerve damage who lives in constant agony and nothing else works). The 40 mg twice a day dosage is equivalent to taking 32 (yes that’s thirty-two) Percocets in a day. This dosage would literally kill the least hardy patients who were not tolerant to these high dose narcotics. Now read the tag line on the ad above, “For moderate to severe chronic osteoarthritis pain”. Huh? Did you read that right? You mean Endo pharmaceuticals actually spent millions pursuing a new indication for this drug so doctors could prescribe the equivalent of 32 Percocets a day for someone with moderate chronic knee pain? Let’s think about this for a second. The vast majority of patients with chronic moderate knee pain would be managed by NSAID’s (Motrin, Alleve, Ibuprofen, etc..), Tylenol, physical therapy, steroid shots, hyaluronic acid shots (SynVisc, Hyalgan, Supartz, etc…). If we take the 19 million U.S adults with arthritis related activity restriction (CDC 2003-2005) and eliminate the 80% that get better or can be managed with these conservative measures, that leaves only about 4 million adults. Since the vast majority of symptomatic arthritis is knee or hip arthritis (joints that can be replaced), we’ll eliminate 2/3’rds of these patients who could get a knee or hip replacement (we don’t like these options very much for many patients, but they are certainly more elegant when compared to high dose, life-long, addicting narcotics). That leaves about 1.3 million adults. Of these remaining 1.3 million adults, the vast majority of those, if they didn’t respond to anything else could be managed by an occasional narcotic like a Percocet, Vicodin, or other similar drug. If we reduce this down now to hundreds of thousands of adults who still can’t be managed, the vast majority of those patients would be managed by regular narcotic use with doses between an occasional Percocet and several a day. Even if we don’t include the patients who would respond to alternative arthritis modalities (such as acupuncture, platelet rich plasma, prolotherapy), we’re still down to at best tens of thousands of people. These patients could of course be managed with any number of high dose narcotics off-label, as many prescriptions for chronic pain are off-label. So why would Endo pursue an expensive additional FDA approved indication for this drug? There can be only one reason, marketing. While doctors can write these high dose narcotics or any drug “off-label” (for a different indication or for a different dose than the FDA approved indication/dose), the drug company is not allowed to market the medication that way to doctors. Now why would Endo want to spend this kind of money (many millions of dollars) to market to doctors to write these Opana prescriptions to a handful of patients who have arthritis and can’t have their pain managed in any other way? They don’t, as the return on investment would be a nightmare. There is only one move here that makes sense from a business perspective (IMHO), market this drug to family doctors and general practitioners for use in the 19 million arthritis patients in the U.S. After all, this number is rapidly going up. The real game plan here is to redefine arthritis management to doctors. The message will be that it’s OK to write a very dangerous and addictive life time drug (once a patient is on a drug like this they generally never get off without a stint in rehab) for patients who could be managed more elegantly in other ways. As a pain management specialist, I find this most recent move by Endo very disturbing. As the ad says above, “Think”…think about why Endo paid many millions of dollars to go after a minuscule market of pain patients.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.