More Crazy Stem Cell Clinic Press Releases for the New Year…
This week I saw yet another crazy press release. I usually see these from some university claiming a stem cell first that isn’t close to a first. Yet now they also seem to be coming from doctors who are claiming firsts that are no where near firsts. This one was from an Orthopedic Sports doctor who claims to be the only orthopedic surgeon in the country using stem cells (we have a few in our network and bone marrow stem cells have been in common use by surgeons for at least a decade). The story was so riddled with inaccuracies (stem cells coming from blood, stem cells not being common practice because of the Bush Embryonic stem cell ban, that there is something unique about a little bedside centrifuge processing bone marrow), that I thought it was time to again differentiate what we do from the wild west of stem cell clinics cropping up.
When we first pioneered orthopedic stem cell therapy in 2005, we were the only physicians in the U.S. doing this type of work. This last few years has seen a bevy of clinics opening up and offering stem cell therapies for pretty much whatever ails you-from arthritis to ALS to COPD to MS. A few of these clinics are legitimately trying to do a good job, but most are not. How can you tell the difference? First, let’s look at the clinic types that are popping up:
The Miracle Fat Stem Cell Clinic-These clinics offer treatments for a multitude of diseases that include knee and hip arthritis. They perform a small liposuction to get cells and as such, are usually run by a plastic surgeon who oversees a processing facility that distributes cells to other medical specialists. Some claim to be operating research studies, but when I have investigated these further, most of this is more sales than reality (i.e. one clinic system claimed to have a research IRB approval that turned out to have been rescinded). In addition, on the orthopedics side of the treatments, these are usually blind non-specific injections (without any guidance to ensure accurate placement) somewhere in the vicinity of the painful joint. They frequently will combine these local injections with an IV infusion of fat stem cells, 97% of which will end up in the lungs and never see the joint. As you know from previous blogs, fat stem cells don’t work as well as marrow cells for orthopedic purposes, so the orthopedic side of the business seems to be an afterthought to drive revenue.
The Little Bedside Machine Clinic-These clinics are often more focused on orthopedic problems, but use an automated bedside “one size fits all” machine to process bone marrow cells and platelet rich plasma. Some of these clinics do offer guidance of the injection, but very little effort is placed on tracking patients or reporting outcome data. So the type of treatment registry data that you’ve read about on this site over the past month isn’t going to be reported by these clinics, leaving the patient to fly blind on how well these procedures work or don’t work. These machines also produce about 1/10-1/15th of the stem cells per unit volume as a Regenexx-SD procedure (based on our lab studies). They also only isolate one fraction in the bone marrow that contains stem cells and discard the other fraction (not knowing that it has valuable cells).
So what key components should a clinic have so you can feel comfortable?
- Treatment registry tracking of patients
- Guidance of the injection
- A focus on orthopedic problems
- Candidacy grading
- Published research
- A customized approach to the processing of your tissue
Treatment Registry Tracking of Patients
Any new therapy that is yet standard of care needs to have data collected, even if it looks very promising from the standpoint of patient experience (i.e. a doctor says it has worked well in other patients). This means that standardized questionnaires are sent to the patient at set time points to see if they have less pain, more function, or had any complications with the procedure. This is a huge commitment on the part of the clinic and the doctor. As an example, right now we have a Clinical Research organization quality customized software to assist us in collecting data on the patients we’ve treated. We have two full-time employees to collect data, several part time supervisors, and a full time bio statistician to analyze this data. When we want to report the data, we must enlist the help of expensive physicians to call patients who haven’t responded to their questionnaires as this helps to make sure we have enough data to report. While we have a bio statistician, we must then use more expensive doctor time to help him decide what’s clinically meaningful to analyze.
How can you tell if a clinic is doing this? They will have data from their patients that they have collected and reported, usually on an annual basis. As an example, the clinic mentioned above with the little bedside centrifuge that claimed magic, had no data and just began doing this procedure, so you wouldn’t expect there to be any. Why is it important to see that clinic’s data? A procedure like this may produce very different results in a different doctor’s hands. In addition, the clinic will be able to tell you exactly how it collects it’s data, who collects it, how often, etc… For example, a proper treatment registry collects data at set time points like 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, etc… If all you get is a call from a nurse like you would after any common surgery, then this isn’t nearly enough.
Guidance of the Injection
How we deliver stem cells as part of Interventional Orthopedics makes a big difference. While delivery into an arm vein (IV) is attractive because of the low level of expertise needed to deliver cells, studies have consistently shown that adult stem cells delivered in this fashion are trapped in the lungs (pulmonary first pass effect). Of even more concern is a recent study showing that for patients considering the use of stem cells to treat Central Nervous System (CNS) disorders, only about 1 in 200,000 cells injected via an IV route reaches the brain and central nervous system (1.5-3.7% made it past the lungs, 0.295% made it to the carotid artery, and 0.0005% made it past the blood brain barrier into the brain). At this point, until these pulmonary first pass issues are worked out, credible stem cell delivery is local. This means placing cells directly into the tissue or into the arterial circulation that directly supplies the tissue. In addition, based on our clinical experience, for orthopedic applications (and likely for others), it’s hyper-local, meaning that placement of cells into one part of the joint may provide results; whereas non-specific placement in the joint may provide less results. This means imaging guidance to place cells into joints is very important. This also means that for an injection based procedure, an orthopedic surgeon is less qualified (without specialized training), as this type of needle based guidance isn’t part of orthopedic surgery training.
A Focus on Orthopedic Problems
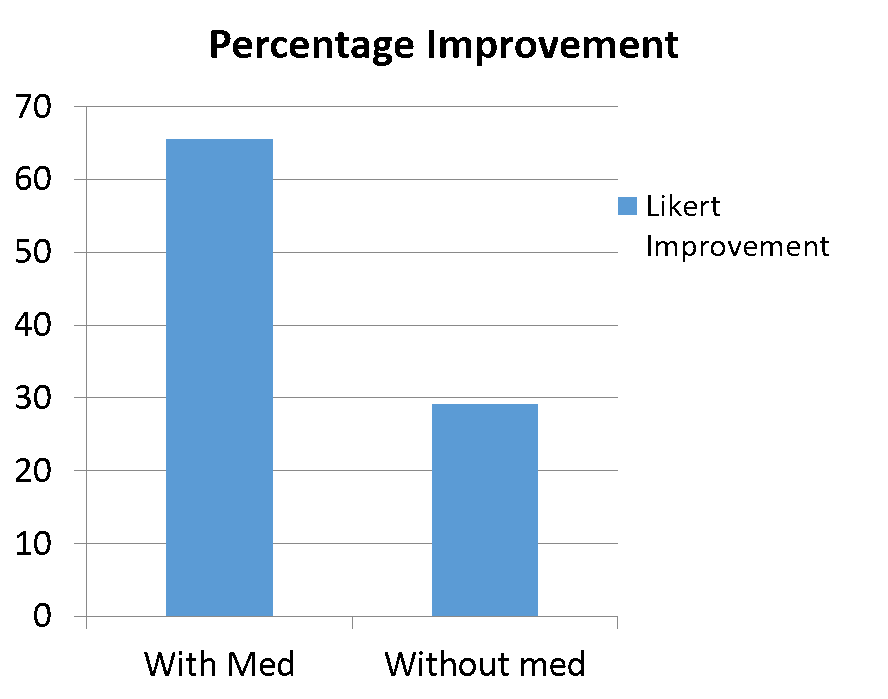
Figuring out how to maximize the effects of stem cells is critical. Let me give you some examples. Early on in our experience, we added in the use of a billionth of a gram of a common medication based on many research papers showing that it helped stem cells create more cartilage. We then had a natural experiment, where we were able to compare patients who didn’t have their cells exposed to this very low dose medication versus those who did. The graph below showed how much better the patients who get the medication did and as a result, this medication became a standard part of our protocol. This is just one example of how little things about how stem cell procedures are performed can make big differences.
As a result, it’s not credible for a clinic to offer therapies for 10 different diseases that have little to do with each other. Credible clinics focus in on one or two body systems and perfect their treatment protocols. This is why we’ve kept our hyper-focus only on orthopedics and why we continue to do the basic science needed to improve our treatments. For a short video break below, click on The Regenexx video link below.
Candidacy Grading
There is no medical procedure available (including stem cells) where all patients are great candidates and expected to do well. Over the last almost decade that we have been offering stem cell treatments, we have graded patients with regard to candidacy. These Good, Fair, or Poor candidate grades have literally dissuaded hundreds of patients who were considered less than stellar candidates from undergoing the procedure. Several years ago, after we had enough outcome data on the Regenexx-C cultured procedure, we lifted those grades as the statistical analysis didn’t show that more severe arthritis patients did any worse than patients with mild arthritis. However, we kept them in place for the Regenexx-SD procedure, as the literature on platelet rich plasma did show that PRP didn’t work nearly as well in moderate and severe arthritis as mild arthritis. In addition, the same holds true for knee micro fracture. Again, we turned away hundreds of patients because adopting a conservative candidacy system that fit with what we knew about regenerative interventional orthopedic procedures and arthritis was the right thing to do. In 2012, our first registry analysis of Regenexx-SD showed that these candidate grades roughly followed the outcome (patients then considered “Poor” candidates with severe arthritis generally had less robust outcomes than those who were “Good” candidates with mild arthritis). So we continued to try to convince many patients with more severe arthritis not to undergo the procedure. In late 2013 we again ran the data with many more knee cases in our registry. Interestingly, as the numbers of patients being tracked increased, the association between severe arthritis and poor outcome didn’t hold up, meaning that the severe arthritis patients who chose to do the procedure anyway had about the same outcome as the mild arthritis patients. So after many years of turning away hundreds of patients, we now feel comfortable in the statement that the Regenexx-SD proprietary knee stem cell procedure and it’s three part treatment process works as well in severe arthritis patients as it does in mild patients.
So ask the clinic you’re evaluating about your candidacy. Is it Good, Fair, or Poor and why? Is anyone considered a poor candidate? For example, I can tell you that if you have knee arthritis and over the age of 55, our data shows that you’ll do as well as someone who is younger. However, based on the hip arthritis data we have right now, if you’re over 55 or have poor hip range of motion, you are unlikely to do as well as someone who is younger with good range of motion.
Published Research
In any new procedure, research should be published as the data becomes mature enough. We have always prided ourselves in submitting our data to peer reviewed journals for publication. This takes an immense amount of work, as any single publication often goes back and forth for months to a year before it’s a form that will appease the journal reviewers. So ask if the clinic has research that they have published. Be careful here, as this is a prime area for bait and switch, as I’ve seen many web-sites from clinics who show research that has nothing to do with their stem cell type or procedure. For example, showing research done by someone else on bone marrow isolated mesenchymal stem cells when the clinic uses fat stromal vascular fraction (an apples to oranges comparison). Ask the question-where is your data on what you do? Ninety nine percent of the time you’ll find that the clinic has no such data.
A Customized Approach to the Processing of Your Tissue
Every patient is unique, yet many clinics use small, automated one size fits all machines to process tissue because the capital and time investment is less. Most of these machines treat every sample as if it were the same, yet every sample is really different. Hence what comes out of the machine is often not processed based on the individual characteristics of that tissue, so the stem cell yields are compromised. Ask the clinic if they process your tissue by hand in their own lab or if they use a small bedside machine that will treat you like a number rather than an individual.
The upshot? There’s a difference between what we do at Regenexx and the crazy stem cell clinic hype press releases popping up. It’s a long-term dedication and almost a decade of experience. It’s spending the extra money on our own research program and a treatment registry. It’s publishing paper after paper and our registry results on-line so we can let patients honestly know if they’re a good candidate or if they should do something else.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.