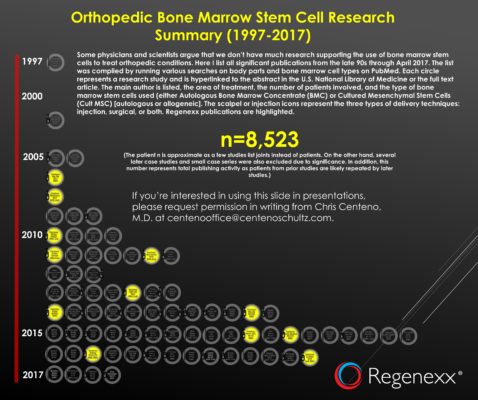
2017 Orthopedic Bone Marrow Stem Cell Research Infographic
Every year for the last four to five or so, I have compiled a list of the published bone marrow stem cell research. Since I’ve updated this every spring, here’s my 2017 edition. Overall, 2016–2017 has been a mixed year for bone marrow stem cell publications. The good news is that we’ve seen higher quality, but still small studies being published. The bad news is that the total publishing volume is down. I suspect this is because we see more diversity in stem cell types being used. This development is likely a good thing.
(Click here for a larger view)
The Summary of What Got Published
The good news is that we see more high-quality research being published for knee osteoarthritis (OA). Meaning, there are more randomized controlled trials or control groups. On the other hand, the total number of patients in these studies is still small at 25–50 patients.
The total number of patients {cumulative patient n) is now up to more than 8,500 people. So the next time someone tries to tell you that we have little research on bone marrow stem cell use in orthopedics, just quote that number. While some would claim that clinical research in orthopedic stem cell use is in its infancy, this body of evidence is reaching late adolescence.
What’s fascinating is that some key trends are emerging that aren’t good for the U.S. Outside of our Regenexx studies and a few small ones out of US universities, Spain has been dominating culture-expanded bone marrow stem cell research in orthopedics. This trend is due in large part to regulatory differences between Spain and the U.S. In essence, our US regulatory structure has hamstrung American orthopedic stem cell publishing. Even more bizarre is that despite outspending countries like Spain 100–1,000 to 1 in basic-science stem cell research, we’re somehow falling behind in the clinical research arena.
The Need to Start Including Other Cell Types in My Summary
What I found exciting these last two years is seeing the growth in the use of other stem cell types in orthopedics. In particular, the use of stem cells derived from fat has been a part of more studies. While last year there wasn’t enough to include to make a go of it, this past two years, there is enough to warrant inclusion. As a result, tomorrow I’ll post an updated infographic that has all cell types listed.
The Percentage of Total Patients That Are Regenexx
Every year I perform a calculation of the total number of patients that we have published on versus the total published. This is a rough metric of our publishing activity versus the rest of the world. I’m proud to report that 49.5% of the total n (patient number) as of April 2017 are Regenexx patients.
How to Use This Infographic
The image above links to a live PDF. Each circle represents a different study and has a live link to that publication. Inside the circle, it lists the first author, the body part treated, the number of patients treated in the study, and the type of cells used. There is also a syringe icon if the study was injection-based, a scalpel if the study was surgical, and both if both implant routes were used.
While I try hard to include all studies, I may have missed a few. Same for making sure all of the links work. So since this is a multi-year project, please ping me via e-mail if you see any issues. The e-mail that goes to my desk is [email protected].
The upshot? We see still see strong research publication in bone marrow stem cell use in orthopedics. Due to enough data being published on other stem cell types to warrant inclusion in my summary, I’ll pull the trigger on a bigger review that I’ll publish in tomorrow’s blog.
4/19/17—Added 2 papers by Mardones.
10/1/17-Added our shoulder paper that I had initially neglected to include.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.