Amniotic Centers of Excellence and Misinformation
Patients reach out to me all the time. This week I was sent a patient-information packet written by an amniotic and umbilical cord product vendor for my thoughts. As I often do, it’s usually easier to write a blog with my opinions and refer everyone back to that document than pen a long e-mail, so here goes.
Amniotic and Umbilical Cord Bait-and-Switch Scams
I have written many pages on the misinformation that commercial amniotic and umbilical cord products contain live and functional stem cells. Frankly, I really wish they did, but then the reality of our lab testing got in the way. Basically, our tests and those performed by an independent third party demonstrated that these products are nonviable, without any living and functional stem cells. See my video below:
A Consumer Guide to Amniotic and Umbilical Cord Stem Cell Therapy
While I’m focusing on one document today, there are many of these around the Internet produced by amniotic or umbilical cord tissue vendors. This one was authored by an orthopedic spine surgeon who lost his license to practice medicine back in 2009, so, regrettably, some of the inaccuracies here are likely due to the fact that this surgeon never performed any common orthobiologics therapies himself, like treating knee, hip, or shoulder arthritis. Meaning lack of personal experience always increases the odds that what you think is valuable information turns out to be misinformation.
I will take the issues with the document one by one in order to educate patients about how easily real information can be misused. I’ll copy the statement and then analyze what’s true and what’s not.
______________________________________________________
“Research performed on the products of conception have shown several benefits to what the
materials contain including:
• High numbers of Stem Cells
• Concentrations of Growth Factors
• High numbers of Cytokines
• Additional elements including exosomes, microsomes, secretomes, mRNA.”
This statement is mostly true but deceptive from the start. The “high numbers of stem cells” part would only be true if you were talking about tissues obtained from a live birth and processed right away in the lab to isolate and then culture expand stem cells, which is illegal in the U.S. However, as our research has shown above (in the video), once you take these tissues, leave them in an OB ward fridge for pickup, transport them across town, leave them in a processing-center fridge, process them, freeze them, store them, ship them in an airplane, ship them in a truck, freeze them in a clinic, and shock thaw them at the bedside for use, you get a collection of dying and dead cells that don’t survive beyond a few hours. This is the type of product sold by this tissue vendor, hence, this statement isn’t likely to be accurate. In addition, as you’ll see below, in order to confirm live and functional stem cells, there are specific scientific guidelines that need to be followed (see FACT guidelines video below).
________________________________________________________
“You may see competitor marketing materials that state “products of conception” do not contain live cells. This is true if the biologics are radiated during processing or contain too much preservative. But it is not true if the materials have been processed without significant radiation or preservatives.”
This statement is also false. Both research teams that produced the study shown in the video above tested only one product that had been irradiated and many that were not. The irradiated product (AmnioFx) didn’t claim live stem cells while the non-irradiated products (BioD Restore, FloGraft, and Ovation) were not irradiated and all claimed live stem cells. None of these products had live stem cells. In addition, our research group has since tested several more products including cord blood, all of which claimed live cells (none were irradiated) and all had no live stem cells. In addition, none of these products contained preservatives other than DMSO, which would be necessary to keep the cells alive as it’s a standard cryoprotectant.
As I have written before, for any amniotic or cord stem cell vendor to prove that their product contains live and functional stem cells, they simply need to have their product tested using the guidelines posted by the Foundation for Accreditation of Cellular Therapy (FACT). None of these birth-tissue vendors has yet submitted to these simple, but rigorous tests. For info on those tests, see my video below:
_______________________________________________________
“In addition, these materials have been shown to work well overall for musculoskeletal conditions, neurodegenerative issues, autism, stroke, organ failure, autoimmune conditions and “tough to treat” issues like Lyme disease.
We have listed several references at the bottom of this guide for review and go to pubmed.com for a comprehensive list by searching.”
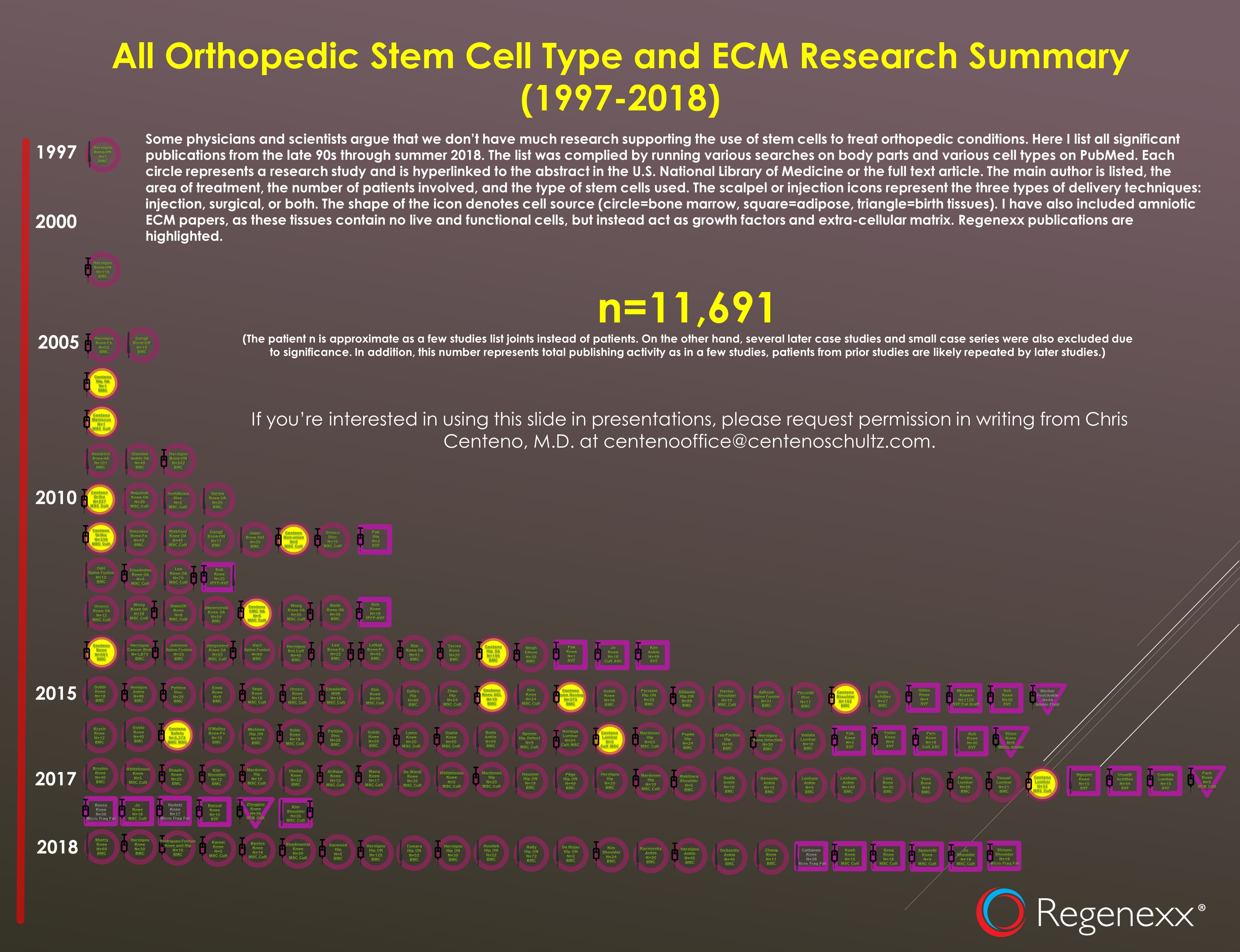
Hmmmm…I just spent many hours looking for all of the research published to date on all different types of orthobiologics including bone marrow, fat, and birth tissues. The extensive infographic I produced is below:
To see this in more detail, click on the image. Basically, each icon (circle, square, triangle) represents one research study. If you click through to the PDF and then click on an icon, it will take you to the research. The circles are bone marrow stem cell studies, and as you can see, most of the research on knees, shoulders, hips, ankles, and backs used bone marrow stem cells. The squares used fat, as we see some of those, but not nearly as many. The triangles are birth tissues, and there are very few of those (only four). Hence, the statement that “these materials have been shown to work well” isn’t supported as there are just a handful of studies, most of which were on foot and ankle.
“There are too many studies to count looking at the effectiveness of amniotic/umbilical tissue to treat musculoskeletal conditions.”
Huh, please count the triangles above as that’s the totality of the literature right now on the use of birth tissues in musculoskeletal conditions. I count four studies, but only two used commercially available amnio products, and the other two foreign studies used US-illegal cultured umbilical cord cells. So I guess “two” is “too many studies to count”? Really?
__________________________________________________________
“The DNA factors are removed to prevent rejection, making the biologic material immunologically privileged.”
Hmmmm…Not sure where to start with this statement. Immune rejection can happen any time a foreign tissue is introduced into the body. DNA is necessary for living cells, so removing it kills cells, and this author is stating that his products have living cells. Hence, the statement makes no sense.
__________________________________________________________
“Bone marrow derived stem cell procedures require an aspiration from the patient’s iliac crest (pelvis). Studies have shown a 29% incidence of chronic pain from the aspiration procedure along with potential for additional complications such as nerve/vessel injury, bowel perforation, fracture.”
Wow, this statement is a real humdinger of misinformation. The 29% “incidence” (the proper term would be “prevalence”) of chronic pain after a bone marrow aspiration (BMA) is nutty. There are two large studies that looked at this issue. One was a UK study that reported only 15 adverse events in more than twenty thousand procedures. The other was our study that looked at all complaints reported by more than two thousand patients who received more than three thousand bone marrow stem cell procedures. We also had a team of independent adjudicators review the complications. None of these patients reported chronic pain at the BMA site. In addition, none had fractures or bowel perforations. This information is also not referenced, so it appears to be pulled out of thin air.
The upshot? As you can see, it takes an immense amount of time for a practicing physician like myself to keep up with the misinformation parade that is the “stem cell” wild west. Each one of these reviews and analysis of the crazy stuff that people put out there takes hours, often between 5:00 a.m. and 7:30 a.m. before I start my clinical day. I can only get to a small amount of the bad information, but I have to say that the thanks I get from my patients as I see them in the clinic are what keeps me writing. So keep sending me crazy info that you find online as my goal is to make sure that my patients understand what’s likely true and what is just plain made up!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.