More Marrow Cellutions Craziness
One of the more interesting gimmicks of the past few years has been a device called Marrow Cellutions (MC). This is a device that purports to harvest bone marrow to maximize the number of stem cells, but every single test performed by the company that compares it against another method or system is fatally flawed. That also applies to the most recent paper by Scarpone, et al. Let’s dig in.
Patients Believe That a Stem Cell Procedure Is a Stem Cell Procedure Is a Stem Cell Procedure…
One of the biggest misconceptions that patients have is the idea that everything called a stem cell procedure is the same thing as everything else called a stem cell procedure. In fact, nothing could be farther from the truth. The masses comparing prices between procedures without doing their homework are often buying a beat-up used Chevy for BMW prices. Let me explain.
Let’s just compare one type of stem cell procedure and focus on bone marrow concentrate (aka a same-day stem cell procedure). The quality of your procedure will be determined by several things:
- The number of stem cells obtained by the draw
- The number of stem and other cells concentrated by the processing
- How your doctor delivers those cells
This morning we’re focusing still further on how many stem cells the draw procedure is able to capture. The problem? Doctors often take shortcuts in this step and thus dramatically reduce stem cell numbers (the beat-up Chevy). Why? The patient doesn’t know the difference, and this saves the doctor time.
Low- vs. High-Volume Draws
In order to fully understand this topic, you need to know a little well-known fact about drawing bone marrow to get the maximum number of stem cells. What you think your doctor should do is actually the opposite. Let me explain.
In a bone marrow stem cell procedure, a trocar (specialized needle) is used to access the bone marrow space. If numbed correctly, this is a comfortable procedure where the doctor draws out what looks like thick blood, which is called “bone marrow aspirate.” The provider’s first impulse is to draw out the most volume he or she can from one spot. However, we’ve known since the ’90s that this actually reduces the number of mesenchymal stem cells per unit volume drawn. Why? Once you get past the first few cc’s, you’re drawing mostly peripheral blood and not bone marrow aspirate. Hence, to get the most stem cells, you need to draw less volume per spot and, hence, you need to draw from many spots. To learn how complex just this one step can be, see my video below:
Up until recently, there haven’t been many different bone marrow aspiration tools. Basically, a standard trocar for this purpose is a needle with a handle called a Jamshidi. There are some that are a bit fancier, and these have holes on the side of the needle. These past few years, we’ve begun to see some innovation in the design of these devices, so now we’re just beginning to see a few more options.
Comparing the Number of Stem Cells Harvested by Different Devices
Now that there are a few different devices, the first job of any good and conscientious doctor is to test them to see if the new devices are any better than what’s usually used. How should this be done? Is there a right way and a wrong way?
When comparing one bone marrow device to another, to test to see if it’s the device that matters, you need to use the same draw technique. If you don’t and you see that one device that used a lower-volume draw technique pulled more stem cells than a device that used a higher-volume one, because of what we learned above, that’s only proof of what we already know—that a low-volume draw beats a high-volume one. Meaning, you can never conclude that the new device contributed anything to the result.
One of the new bone marrow aspiration devices on the market is called Marrow Cellutions. It actually has many different names. For example, it’s also resold under the brand name Maxx Regen. So how does it stack up? Regrettably, all of the research published by the company on this device falls into the trap I described above. The company uses a low-volume draw technique known to get more cells and compares that to the standard device using a high-volume draw procedure know to yield fewer cells. Basically, the company produces research comparing apples and oranges and then tries to convince the physician that their apple is better than the orange.
Testing the Marrow Cellutions Device
We tested this device a number of years ago. Why? Because if it really worked as advertised and got more stem cells, that would change what we did. We would then further concentrate those cells in the lab as we do now. After all, what’s not to like about more cells?
We bought several devices from third parties after the company refused to sell us product (they knew we would be testing them). This is what we found:
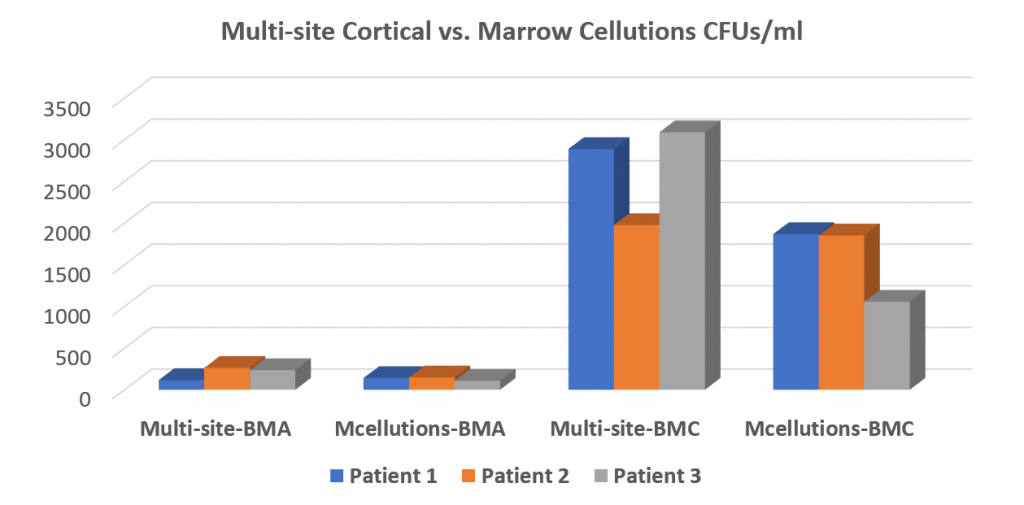
The height of these bars represents stem cell content. The key comparison is the second set of bars (MC device) compared to the third set (what we typically produce for our same-day stem cell procedure using a Jamshidi trocar). As you can see, the device badly underperformed.
The Past Marrow Cellutions Papers
The company that makes the Marrow Cellutions device has produced a number of different white papers. I have blogged on them in the past. Basically, all had fatal flaws that didn’t support their conclusions.
- 2017 Marrow Cellutions white paper—update
- Initial Harrell Marrow Cellutions paper
- 2018 lecture series by Andrew McGillicuddy on Marrow Cellutions device
Unraveling the Most Recent Paper
After publishing a spectacularly mind-insulting series of papers, you would think that the company that makes the Marrow Cellutions device would have gotten the memo. The only paper that will count, is one that compares the same low-volume draw technique used with the MC device to the usual Jamshidi trocar using the same low-volume draw technique. However, despite knowing that’s what they need, they produced this new paper, which, like all of the others, isn’t worth the paper it’s printed on. Let me explain.
In the methods section of this new paper by Scarpone we see this: “In comparison 1, the SSLM method was compared to Harvest BMACs derived from three puncture sites. In comparison 2, the SSLM method was compared to Harvest BMACs derived from a single puncture site. Finally, in comparison 3, the SSLM method was compared to EmCyte BMACs derived from a single puncture site.” Since the Harvest system typically takes 60 ml to operate, that means we’re comparing very high-volume draws to one low-volume draw, which makes the paper’s conclusions null and void.
The company’s defense? It used manufacturer’s instructions for these devices. Huh? That’s like saying that the manufacturer specified fruit and we chose to compare apples to oranges. It has also been brought up that the manufacturer’s claim in the 510K device clearance for the MC device is that the device is the same as the typical Jamshidi. Go figure.
How Can They Keep Selling This Thing?
The MC device, to me, is the worst example of what happens in medicine with marketing driving the bus. Meaning, once we and other independent labs published our results showing that the device didn’t work as advertised and once the company kept producing bad research, physicians should take note. However, they keep selling it. How does that work?
First, most physicians have no or very little knowledge of this area. Orthobiologics as a subject isn’t taught in medical school, residency, or fellowship programs. Second, most of the conferences in this area of medicine are paid infomercials and not objective sources of information. Third, most physicians get their training in regenerative medicine from sales reps. Finally, we have the phenomenon of KOLs.
A KOL is device- and pharma-company speak for a “key opinion leader.” These are basically paid spokespeople who are doctors. These doctors can sway the opinion of physician purchasers.
Does This Thing Work Clinically?
There’s another possibility. Despite the company’s insistence on incorrectly comparing the stem cell content of what the device produces, this thing may work to help patients for other reasons. I can’t rule that out. However, despite knowing that what this device produces is different from bone marrow concentrate (it has fewer stem cells), so far we have zero clinical publications on how it works in real patients. Even if someone were to publish outcome data tomorrow, the company would be decades behind what’s been published already on BMC.
The upshot? Does the Marrow Cellutions work to increase stem cell yields? Not as far as I can tell based on our own analysis and those published elsewhere. In addition, the company has published no compelling research that supports this idea. Does this device work as well clinically as bone marrow concentrate? There’s nothing published, and it’s unlikely that we’ll see anything anytime soon.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.