The CFU Game
One of the more interesting things I’ve seen in regenerative medicine is how physicians with no direct experience in working with a research lab can often get in trouble with a little bit of knowledge. One of the areas where this happens is in quoting a simple stem cell quantification number that goes by the acronym CFU. This misunderstanding of how this metric works has caused a bunch of bad device white papers to be published that have inappropriately influenced physician decision making. Let me explain.
What Is a CFU?
When using bone marrow or fat-based stem cell treatments, there is no easy way to get a “stem cell” count. Meaning, you can’t look under a microscope or use a simple bedside machine and get this number. You can count the total number of cells (total nucleated cell count, or TNCC). Given that the stem cells are some fraction of these cells and that total cell number roughly correlates with stem cell number, this is a reasonable proxy. However, getting to the actual number of stem cells in a sample is more complex.
You can use a technology called flow cytometry that stains the chemical markers on the surface of cells, but without a single unique mesenchymal stem cell marker, getting a count requires a number of tests of many different markers. In addition, setting up and running a flow cytometer can be so complex that even experienced research scientists often farm this measurement out to a department within a university (often called the “Flow Core”). Hence, while we have two flow cytometers in our Colorado HQ for Regenexx, these expensive and complex machines are not common in doctors’ offices or most hospitals.
Finally, we come to the topic of this post: the commonly used and often abused CFU measurement. CFU stands for colony forming unit. To get this measurement, you take the cellular product (like bone marrow) and place it in a specialized monolayer culture flask. The adherent cells (which are mostly mesenchymal stem cells) will grow on the surface of the flask over two weeks. You can then stain those cells and count the colonies that formed as a very rough metric of the total stem cell content of the sample. Each one of these colonies is called a CFU-f. So the higher the CFUs, the greater the number of stem cells in your original sample. Check out my video below to see what this looks like:
Why a CFU Metric Is a ROUGH Count of Stem Cell Content
How many CFUs you get to grow can be impacted by many things, which is what makes the metric solid for a research study performed at the same lab using the same technique, but makes it awful for comparisons between labs or institutions. For example, you can vary the total number of cells placed in the flask, which is called plating density. So if you plate more cells, sometimes you can get more CFUs. In addition, certain plating densities on the low side can provide more room for cells to grow and spark even more growth, causing a higher CFU count despite the same number of stem cells in the sample.
This plating density issue is a real problem. Take, for example, CFUs obtained in our lab. The same sample was used, so they have the same stem cell content, but notice that the final CFU count (yellow highlight in the red box) for the top graphs is 49.5 while the bottom one is 75. How is that possible? The bottom one was plated at a lower density and these cells had a higher growth rate (they were from a young donor), so the cells had more room to grow. So just changing the seeding density created a dramatically different CFU number.
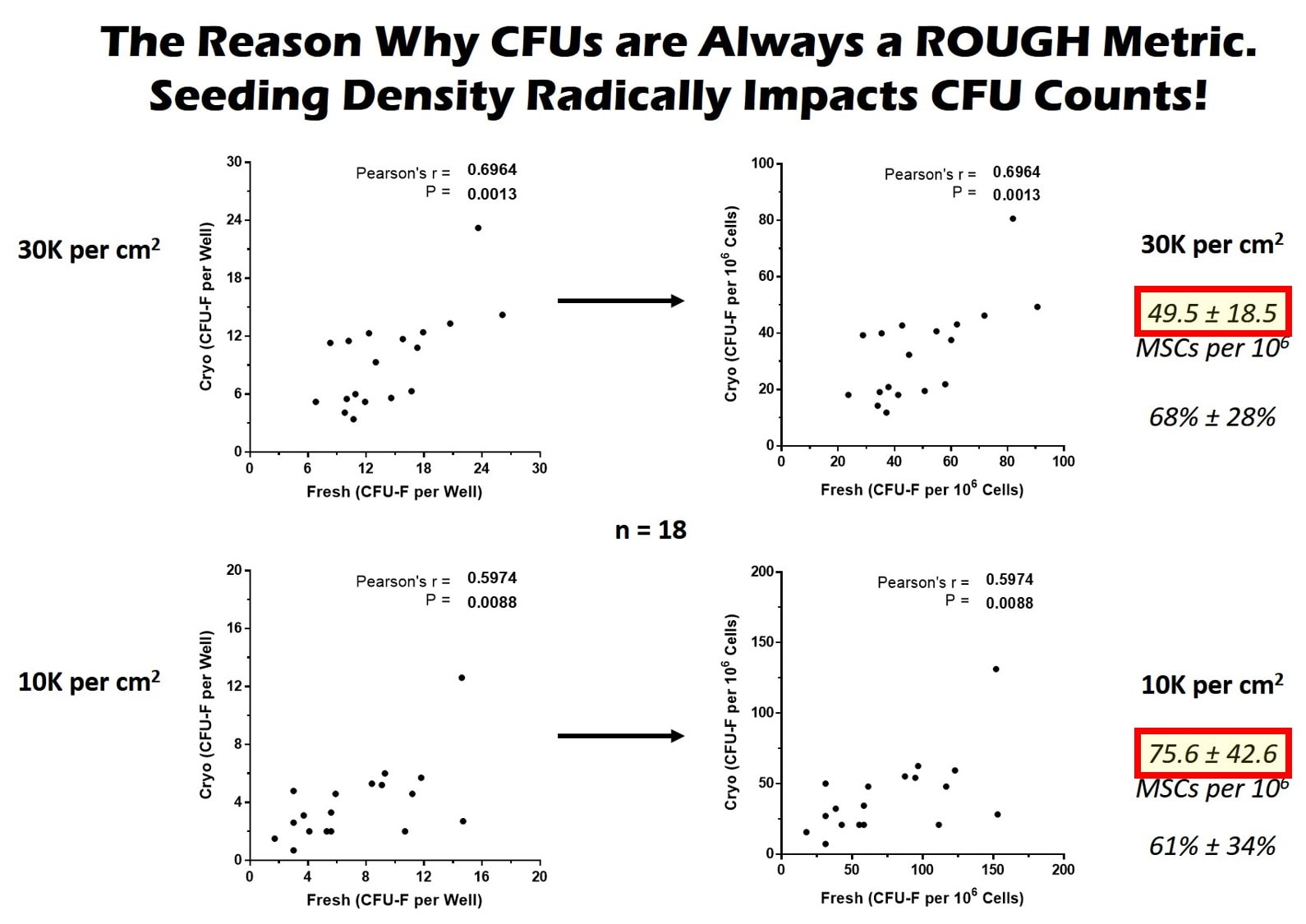
Another difference between labs can be the quality of the growth media used or the length of time the cells are grown. Even the oxygen level in the air and humidity can all make a difference. Hence, within one lab that does everything the same, a CFU count can be a nice and reliable metric to get a rough idea of the stem cell content of bone marrow or fat. However, because of all of these differences, CFUs cannot be used between labs. So one paper that says that Dr. Smith got X CFUs at ABC Lab can NEVER be compared to Dr. X who got Y CFUs at Acme Lab.
The Device’s Industry CFU Problem
Device manufacturers and the physicians who work for them as “key opinion leaders” (KOLs) often badly mangle the conversation around CFUs. As you’ve learned today, while one lab that uses the same technique can compare CFU counts internally for a research study, you can’t compare CFU counts between labs. Hence, when a machine manufacturer claims that they had so many CFUs per unit volume in the bone marrow concentrate that their machine produces, that’s not a number that can be reliably compared to the CFU count of another machine or device.
One of the biggest abusers of this concept has been a company called Endocellutions that makes the Marrow Cellutions device. Their KOLs have published numerous white papers that try to compare the CFUs obtained with this device to the CFUs obtained by bedside centrifuges. This is a garbage in/garbage out comparison for all of the reasons stated, as the CFUs were grown at different labs. In addition, many of these KOLs have compared CFUs obtained by machine manufacturers where nothing was reported about the patient donors! This is double insanity, as if the Marrow Cellutions donors were younger and the bedside machine donors were older, this would be another factor that can dramatically impact CFU counts.
I’ve also seen lecturers who should know better make this same rookie mistake. They get up on the podium or in a webinar and quote CFUs from someone else’s research studies and compare those to CFUs they have obtained. Again, these are all junk science comparisons.
Our CFU Research Project
Recently, our Colorado HQ started the world’s first orthopedic treatment research biobank. This means that we are saving a sample from every treated patient who receives our more advanced bone marrow concentrate. We freeze these down and then bring them out of cryopreservation when we want to run a metric, like CFUs, for a research study. However, figuring out the conversion metrics from fresh to frozen has been a task that’s taken the better part of a year and hundreds of lab hours. Given that no one else has ever done this before, we will be publishing this data for everyone to use and to benefit the field.
The upshot? CFUs are a NOT a metric that can be compared between labs and used like a cell count. If you’re doing that as a doctor or sales rep, PLEASE STOP. CFU numbers can be used for research purposes only when those numbers are obtained from the same lab. Hopefully, through this blog, everyone will get the memo!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.