What Is NeoCyte? Just Another Cord Blood Product?
This has been a bad month for cord blood and birth tissue vendors. We’ve seen multiple cases of bacterial contamination. In addition, a university scientist echoed our earlier research that these are dead birth tissues being hawked as live stem cells. Now, I’ve finally learned more about the cord blood product NeoCyte, which is featured in the IMAC investor packet I drilled down on a few weeks back.
Amniotic and Cord Blood Craziness—Regulation
There are so many layers to this national scam that it’s hard to know where to begin. Hence, let’s begin with the regulations. Manufacturers of birth tissue products, like amniotic membrane and umbilical cord blood, are only required to register with the FDA. This is a free 45-minute listing on the agency website and not an “approval.” Most concerning is that FDA regulations make it clear that if you claim your product has live cells, registration with the FDA is insufficient. Instead, if the manufacturer claims that the umbilical cord product contains live cells, the product will be considered a drug subject to FDA’s full-blown approval requirement, including multiple phases of clinical trials for each indication. To understand this better, see my video below:
So if you claim that your umbilical cord product has live cells, whether or not that claim is true, then your product is a drug, and that product must be “FDA Approved” through expensive clinical trials that take years and often tens to hundreds of millions of dollars rather than a simple and free registration on a website.
IMAC and NeoCyte
IMAC is a chain of chiropractic clinics that advertises that it performs “stem cell” therapy. In 2018, IMAC acquired a company owned by Ian White, PhD, called BioFirma. This company produces a product called NeoCyte. Up until now, there hasn’t been much on NeoCyte, but an investor presentation sent to me by a physician local to the IMAC centers has more detail.
What Is NeoCyte?
Page 21/223 of the December IMAC Investor Prospectus states this about NeoCyte: “BioFirma was formed to produce and commercialize NeoCyte, an umbilical cord-derived mononuclear cell product following the FDA’s current Good Clinical Practices (or cGCPs) regulations.” So it’s clear that this product is what I would term “umbilical cord blood.” Thus it joins the ranks of similar products from Liveyon and the Utah Cord Bank. In fact, these are the products that NeoCyte compares itself to in the IMAC investor slide deck (page 17).
A Word on Viability Testing
Most medical providers don’t understand the finer points of viability testing. To them, if a company says that two-thirds of its cells are “alive” on this type of test, they believe that this means these cells are alive and fully functional. However, nothing could be further from the truth. Let me explain.
There are many types of cell-viability testing that all answer the same question, but to varying degrees of accuracy. That question seems simple: “How many cells are alive?” However, it’s not so simple. Take, for example, your local hospital. If you ask that same question about the patients in that facility, the 18-year-old with a laceration requiring stitches in the ER is placed in the same “alive” category as the 84-year-old on life support in the ICU or the 54-year-old woman clinging to life after a serious car crash. So a better question is likely this: “How many are alive and fully functional?” Now the kid in the ER is differentiated from the old guy and barely alive woman in the ICU.
The simple “live/dead” stains used by a company selling umbilical cord or amniotic tissues ask the simple “how many are alive” question. If that number is in the high 80s to low 90s, then you likely have nothing to worry about. The problem is that when that viability number is less than 85%, you need to seriously worry about the health of the cells. For more information, check out my video below:
NeoCyte and Cell Viability
The IMAC investor presentation provided our first peek into the marketing of NeoCyte. In that slide deck we see this slide:
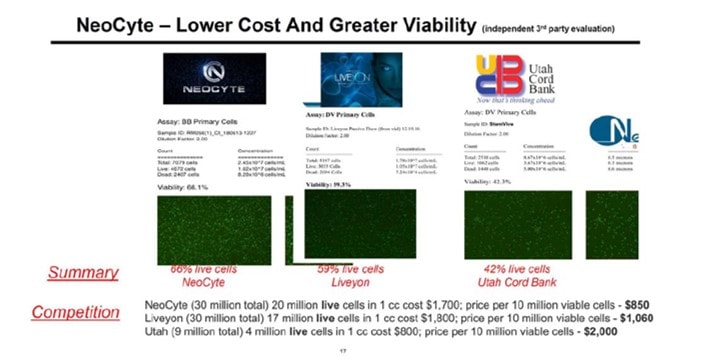
What do we see? First, IMAC is claiming that NeoCyte has viable cells, which would make this a biological or cellular drug that requires express permission from the FDA (typically in the form of an approved IND with a BLA) before the product can be studied on human subjects in the U.S. Thus, due to the claim of “live cells,” NeoCyte’s registration with the FDA would be inadequate: instead, it must go through years of testing in clinical trials per clinical indication. Second, the viability of NeoCyte, despite being reported here as 66% and better than competitors, still leads to serious concerns of whether the product contains any live and functional stem cells. Why? As both our lab and that at Cornell have found out, despite these claims from manufacturers, independent scientific investigations into these products show that they are dead tissue that does not contain live and functional stem cells. See the video below that highlights a recent statement from Lisa Fortier at Cornell on this topic:
NeoCyte and a Patient Result?
I saw this slide (below) in the investor presentation. What is this? First, on the right, it appears that this is a patient result after using an IV dose of NeoCyte for one week. I first checked the FDA’s tissue registration system (accessed 2/10/19 at 4:03 p.m.) to see if NeoCyte is a registered product or BioFirma (or IMAC) is a registered tissue establishment, which would be required if an allogeneic tissue were being used in a real patient. I did not find any such registration for this product or company. I also found no evidence that an FDA-approved clinical trial is active. Why is that critical? It is not legal to use an umbilical cord product in a patient that isn’t FDA registered through the PHSA (aka 361 tissue registration) and also not legal if you claim live cells without an IND/BLA in place.
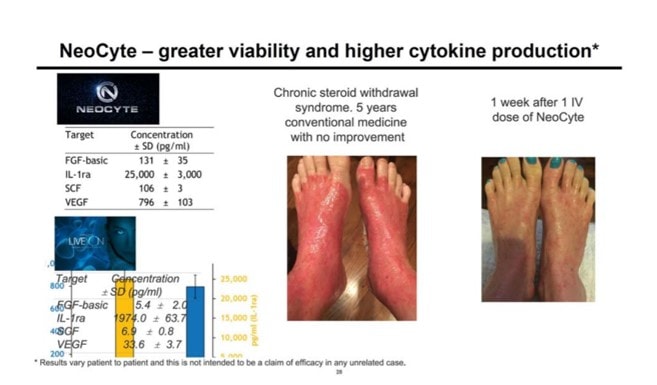
NeoCyte and the Umbilical Cord Blood Industry
The FDA seems to have begun a crackdown on the out-of-control umbilical cord blood and amniotic tissues industries. In a letter on the contamination of the Liveyon product, it stated specifically, “To lawfully market these products, an approved biologics license application is needed. While in the development stage, the products may be used in humans only if an investigational new drug application (IND) is in effect. However, no such licenses or INDs exist for the Genetech-processed, Liveyon-distributed products.” In other words, if you claim your umbilical cord product has live cells, it’s a drug. In addition, it discussed that it was sending out letters to many manufacturers.
So is there a difference between NeoCyte and the Liveyon product that was called an unapproved drug by the FDA? Here are the similarities:
- Both are umbilical cord blood products registered through the 361 pathway
- Both promote that they contain live cells
- Both make treatment claims
Hence, reading the FDA letter, both are drugs requiring full FDA approval with clinical trials BEFORE use.
The upshot? NeoCyte appears to be just another umbilical cord stem cell product claiming live cells, which is not permitted. Based on the viability numbers reported, it appears to be the same old song and dance. Meaning that it’s very unlikely that this umbilical cord product has any live and functional stem cells. Finally, it’s alarming that despite no FDA tissue registration or approvals (that I can find) that the product appears to have been used in patients.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.