Yet Another Marrow Cellutions Review…
Last summer we completed our internal lab testing of an expensive trocar that claimed to be able to concentrate stem cells as the marrow was drawn. The device didn’t perform as advertised, and I had hoped to never write about it again. However, since then, new data has been published by the company, so it was time to refresh my last review.
The Bone Marrow Draw—Understanding What This Is All About
Any bone marrow stem cell procedure begins with a draw procedure where an aspirate is harvested. This procedure involves numbing the area at the back of the hip and then using imaging guidance to draw out what looks like thick blood. The number of stem cells you get depends on how the draw is performed. That last part may surprise some patients, as most would believe that there is some standard way this is done and how the procedure is performed has little bearing on the outcome of their process, but they would be wrong.
First, drawing bone marrow to maximize stem cells is a paradoxical affair. Meaning, you would think that the more volume you draw, the most stem cells you get. Not really. What actually happens is that there are many more stem cells in the first few ml’s of the draw than there are after that point. Why does this happen?
The problem is that mesenchymal stem cells live locally and are usually stuck to the bone and blood vessels. In addition, the entire bone marrow space communicates with the peripheral vascular circulation. Hence, when you draw that first small volume after the needle is in the bone marrow cavity, you pull off the local stem cells from where they live. After that, you’re mostly just drawing blood as you would from a vein, and blood is stem cell poor.
Despite this fact, 95% of doctors performing a stem cell harvest do it wrong. They insert the needle and then draw a large volume, which ensures that the draw has few stem cells. What they should be doing is inserting the needle and then pulling only a tiny volume and then repositioning the needle to a new spot and drawing a small volume and then repeating until they get the desired amount. The problem? The latter type of draw is too time-consuming, and patients rarely know the difference, so the doctor can substitute his convenience at the expense of the patient’s procedure outcome.
To learn more about this topic, see my video below:
The Marrow Cellutions or Maxx-Regen or Ranfac Device
Doctors often concentrate bone marrow by centrifuging it and then separating out the fraction that has the stem cells. Most doctors use bedside machines to do this that frankly leave many cells on the table and discarded, so we have always used our own processing protocols and a flexible lab platform. To learn more about what we do with a centrifuge and why it’s different, see my video below:
Despite these differences, a number of years ago a company came out with a very expensive trocar to draw bone marrow. Usually, a simple Jamshidi needle is used, but this one, because of its unique design, claimed to be able to skip the centrifuge step. This, of course, would be a huge time-saver, if it were true. Hence, we set out to test the device. Why? First, we don’t believe the claims of any company selling us a product and always independently test those claims. Second, if this were true and we still further centrifuged our bone marrow after drawing it with this device, we could get even more stem cells to help our patients.
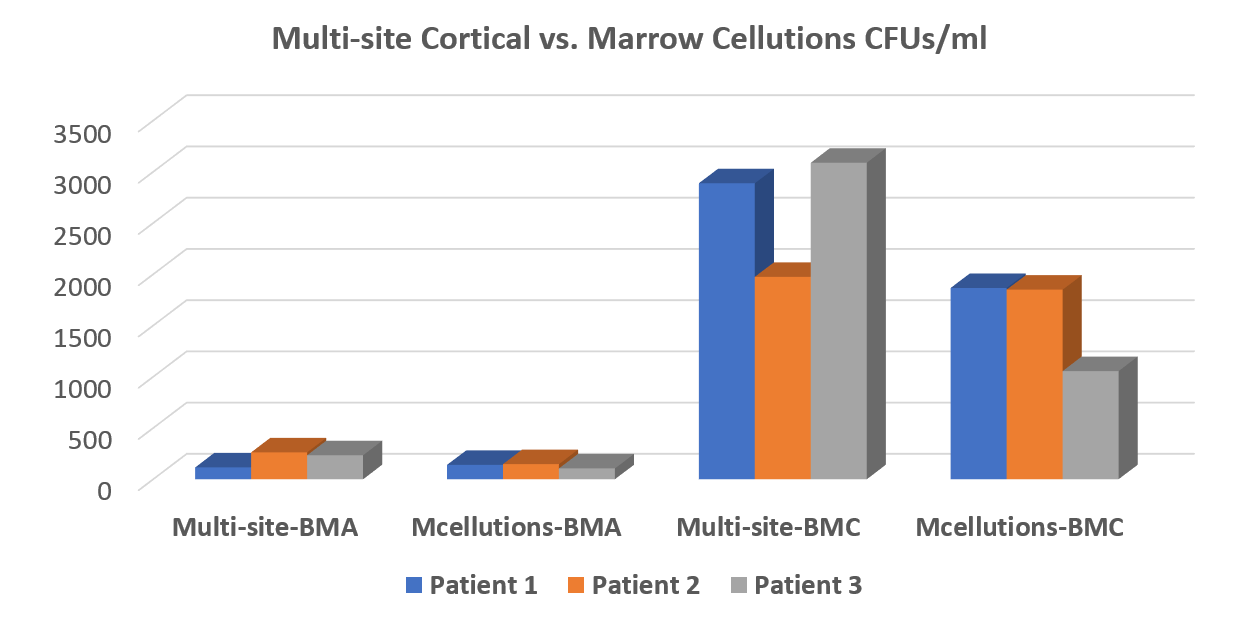
Hence, in the summer of 2017, we tested a few of these devices. What happened? In a properly designed head-to-head comparison where we as doctors blinded our on-site research lab as to which sample was which, the device failed miserably. The results are below:
What’s shown? The height of the bars is the stem cell content per unit volume. The comparison is between the second set of bars (the device alone) and the third (a Jamshidi needle plus our concentration protocol). As you can see, our method smoked the device. In fact, the results were so bad, we decided that purchasing the device for our network sites made no sense.
I’ve also summarized all of this in a video:
The New Data
Companies that are trying to sell devices will frequently produce white papers to show that their doohickey is better. This one was published in 2017, likely shortly after my blogs on the topic. It has all of the same problems as all of the Marrow Cellutions white papers I have seen, and, in short, it claims to compare apples to apples but compares apples to oranges.
The white paper in question purports to compare the Marrow Cellutions (MC) device to a bedside centrifuge made by Emcyte. While I’m no fan of simple bedside centrifuges, this document claims that the MC device outperformed the centrifuge and produced more stem cells. However, this only happened because of some sleight of hand performed in this small study.
Remember where I said that how you draw the marrow is critical for producing high stem cell numbers? Well, the MC device makes you pull the marrow very differently. It requires that you draw small volumes from many spots, and it’s this method that produces more cells. However, the manufacturer of this expensive device wants you to believe that it’s the device and not the method. Hence, they never test the device against less costly trocars or needles using the same type of low-volume draw procedure. When we did that, the device didn’t work as advertised.
For this white paper, the authors compared the MC device on one side of the pelvis to a large-volume draw on the other side. This is not an apples-to-apples comparison, as you now know that if we draw larger volumes of marrow, we get fewer stem cells. The MC device makes you draw smaller volumes from more sites; hence, we would expect more stem cells per unit volume. This is what this advertising white paper found. Again, what should have been done was to compare the MC device to the other side with the same small volumes being taken from multiple sites.
Hence, to use a common-sense example, this paper purports to be about the sweetness of apples but then tests the sweetness of apples versus oranges. It then somehow concludes that the manufacturer’s apples are sweeter than other manufacturers apples, but it never tested against any other apples. Basically, the white paper is more sleight of hand than science.
The Doctors Using This Device Have Another Problem—Reality
If a doctor has decided to use this device, since it produces something utterly different from bone marrow concentrate (BMC), the doctor has a problem. There’s quite a bit of research published that BMC (a same-day stem cell procedure) can help orthopedic issues (much of that published by our group). All of that research doesn’t apply to what you’re doing with the MC device. Hence, you need to be honest with your patient that you’re trying a new procedure for which no published clinical data exists.
Given that the MC device produces a stem-cell-poor product compared to a best practices bone marrow harvest and concentration through centrifugation, this is yet another problem. Why? Multiple studies have shown that the successful outcome of these procedures is tied directly to the stem cell content of what’s injected. For more information on that issue, see my video below:
The upshot? There continues to be no compelling data that the Marrow Cellutions device works as advertised. In fact, we showed that this expensive device worked no better than a cheap trocar used the right way. So, we’ll still steer clear of this device!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.