The Final Marrow Cellutions Review
This past winter, while I was at the Interventional Orthopedics Foundation (IOF) conference, a sales rep for the Marrow Cellutions device approached me. The device had been on our radar for years, but a veterinarian who presented at the conference thought highly of it, so I was intrigued again. That began an ordeal of testing, and now that chapter has ended with the completion of our final tests. So what is this device, how does it work, and what did we find?
Bone Marrow Aspiration
The Marrow Cellutions device is designed to improve the stem cell yield in a bone marrow aspiration (BMA) procedure. A BMA is when a physician numbs the area at the back of the hip and using either fluoroscopy or ultrasound, places a specialized needle to draw out what looks like thick blood, the bone marrow aspirate. The technique has been performed for many years using what’s called a Jamshidi needle. The design of this simple device has a few variations, but suffice it to say that there hasn’t been a major upgrade to the actual needle design in half a century or more. Hence, when a new design hits the market that claims to be able to obtain dramatically more bone marrow stem cells, we take notice. After all, what’s not to like about more cells?
Houston, We Have a BMA Problem…
Being the first physician in the U.S. to use stem cells to treat common orthopedic problems, I have seen how BMAs have evolved over the years, and it isn’t pretty. We’ve known for many years how to optimize the number of stem cells in this procedure, but somehow that has become bastardized into what’s more convenient for the doctor. For example, instead of cannulating many sites and drawing low volumes, which increases yield, physicians have taken shortcuts by only drawing from a single site, which reduces yield. Hence, when a new device comes on the market to improve the stem cell numbers in a BMA, it’s usually compared to the bastardized and “easy for the doctor” draw technique and not a best-practices procedure. See my video below for more info on this topic.
It was in this context, a few years back, that we tested the Marrow Miner. This is a device that takes small samples of bone marrow at various spots as it travels through the pelvis. Regrettably, it didn’t really result in any higher stem cell yields (mesenchymal stem cells) than our standard-of-care BMA. While the Marrow Cellutions device is similar, there are a few differences, so we wanted to test this as well.
Why Test at All?
Almost all physicians now working in this space don’t have any lab capabilities. Hence, they have no way to check the claims of device manufacturers. So they tend to believe whatever data is thrown at them. This is a real problem in the regenerative-medicine space as we have many doctors who don’t know what they don’t know. One example of this is a doctor in new Jersey who claimed that his MC device allowed him to get many more stem cells less invasively and more economically. So was he right or wrong? Let’s review the data…
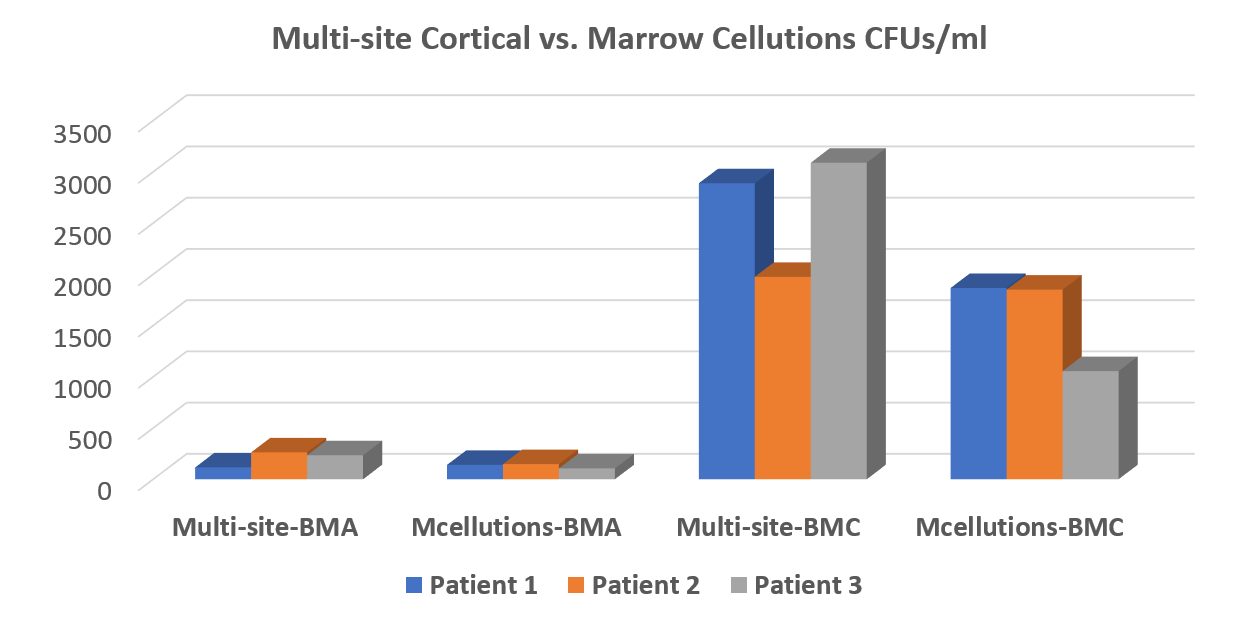
The 30,000-Foot View of the MC Data
The bottom line is that the MC device performed no better for total nucleated cell count (TNCC) and colony-forming units (CFUs—shown above) than a $30 trocar used in a best-practices way. In addition, we couldn’t confirm the company’s claims that the device harvests as many or more stem cells that can be produced by concentrating bone marrow (BMC). In fact, our advanced version of BMC decimated the MC device in all tests. It wasn’t even close. The data is above.
For ease of reporting, I have focused on CFUs. All of the data has been posted to the Biologic Orthopedic Society LinkedIn discussion group for reference. We performed all tests by randomizing which side of the pelvis was assigned a best-practices draw and which was to get the MC draw. The MC draw was performed per the manufacturer’s white paper (they refused to send a rep, but that’s another story). Each draw was performed with a 10 cc syringe with the same amount of heparin, and each site was preheparinized in the same way. The samples were sent to the lab for processing in a blinded manner.
Where Did the MC Story Come Off the Rails?
On the one hand, I like the MC device because it forces you to have impeccable BMA technique. The device requires that you draw small volumes while you slowly and precisely pull it out of the marrow space. On the other hand, you can replicate this with a much cheaper $30 trocar that costs one-twentieth as much. In addition, it’s likely that the MC device is leaving the subcortical MSCs on the proverbial table, as the multicortical site draw performed just as well. My theory is that the multicortical site draw pulls more MSCs that live just below the cortex while the MC device leaves those cells in the marrow space.
If the MC device were sold for about 2–3X the cost of a Jamshidi trocar, it might be worth it for some physicians who don’t want to take the time and energy to learn how to perform a proper multicortical site or low volume, deeper draw. However, at a cost of $600–$1,900, there’s not a very compelling argument that the device is needed. Hence, the company seemed to change their marketing plans from a better trocar to a device that replaces the need to concentrate the cells. While this justifies the higher price, our data didn’t support the idea that the device produces so many cells that concentration isn’t needed. In fact, the device couldn’t even beat the multisite draw without concentration. Ultimately, this is where the MC story comes off the rails.
Practical Considerations
The New Jersey MC doctor above had claimed that the MC device was less painful. That was not our experience. In fact, all three patients reported more pain with the MC device both during and after the MC procedure. Why? I can numb the cortex of the bone and thus patients don’t feel as much. However, I can’t numb the marrow space, and the MC device travels farther in the marrow space.
This Isn’t Just My Data
Don Bufford, MD, a Dallas orthopedic surgeon, also tested this device and reported similar results, so there is corroboration of our data. Why did the company data show completely different results? As I have opined on LinkedIn, I can see that if you used a poor bone marrow aspiration technique and used a machine that was less adept at concentration, you could get results that would show that the per-volume number of stem cells in MC would be higher. That’s not because of any magic in the device, just the magic in the technique it forces you to use. In addition, there may be other confounders. For example, I can’t tell whether the MC white papers showing higher yields inadvertently concentrated the MC aspirate by removing the buffy coat before plating for CFUs, as that’s common practice. In addition, while the per-cc number of stem cells may be higher in the first 1–2 ml of a BMA at any site, even if that yield drops off to half in the 3rd–15th milliliters of a draw, you can still easily overwhelm the total number of cells by drawing higher volumes.
The upshot? While I’ve taken a few hits on LinkedIn from MC-device sales reps for finding results that didn’t agree with their white papers, I really did hope this thing would work. As I’ve said, what’s not to like about a device that gets many, many more stem cells from the patient? That’s always a good thing. In the end, our New Jersey doctor was like most, in that he didn’t know what he didn’t know. In the meantime, we’ll continue our standard-of-care draw.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.