Houston, We Have a Fat Problem: Orthopedic Surgeons and Lipogems
This weekend I saw a local advertisement by an orthopedic surgeon that mangled the facts about stem cells and, in particular, about fat grafts, but it could be an ad in any one of hundreds of local newspapers all over the country. The problem is that we now have some orthopedic surgeons using a fat-processing kit known as Lipogems and, against the recommendations of the company, falsely advertising this as a “stem cell procedure.” So let’s explore the advertisement and explore the bigger problem of the fat graft/stem cell bait and switch.
What Is Lipogems?
I’ve already blogged a detailed Lipogems review, but suffice it to say that this is a kit where the surgeon places fat obtained from liposuction, shakes it in a cylinder that contains several steel balls and saline, and then injects or uses this macerated and cleaned fat surgically. It’s important to note that the device has a simple and quick FDA 510K approval. This merely means that it pointed to a device that cleaned fat for surgical grafting that existed before the FDA began to regulate devices in the 1970s and made the claim that its device was substantially similar to that device. Meaning the company has no approval to make any claims about what the fat graft can or can’t do as far as healing, only that it has a kit that macerates and cleans fat.
Our testing earlier this month revealed that this wasn’t a stem cell procedure, but a fat graft. The company also is very clear that it’s not a stem cell procedure, but a surgical fat graft. To learn more, see the link above or watch my short video below:
What’s the Difference Between a Fat Stem Cell Procedure and a Fat Graft?
A fat stem cell procedure and a fat graft are two completely different animals. A fat graft has cells that are trapped in their tiny collagen prisons, that are unable to escape. Meaning that if you place a fat graft in culture, the stem cells generally perish before being released from the fat. This is why fat grafts used cosmetically have a relatively short half-life. The cells within the graft aren’t all that viable and able to engraft with the surrounding tissues to survive long-term. However, a true fat stem cell procedure uses an enzyme to digest the collagen prisons away, and this allows the cells to be free to act. There’s just one problem: this type of fat stem cell procedure (known as SVF, or stromal vascular fraction) isn’t legal in the U.S. To learn more about the differences between the two procedures and why a fat graft isn’t a stem cell procedure, see the video below:
The Local Orthopedic Surgery Ad: Misinformation Abounds
As I’ve said, the inaccuracies and misinformation in this local ad aren’t endemic to my small town but are being repeated all over the Internet and across local papers all over the country. Realize that all of the misinformation springs from the fact that what’s being offered is a Lipogems fat graft and NOT a stem cell procedure. For example, as you’ll see below, some of the statements made would be true or partly true if the physician were using a bone marrow stem cell procedure, but none of these claims apply to the Lipogems kit being used.
So let’s take this website apart and analyze:
- There are more stem cells in fat than there are in bone marrow… This is basically a fourth-grade math error. In another spot in the local website, it states that there are 500 to 2,000 times more stem cells in fat than in bone marrow. Neither statement is true. To understand why this isn’t accurate, see my video below:
- “…the more stem cells, the better the results.” While this seems to be true based on the early research for orthopedic conditions treated with bone marrow stem cells, it has not been demonstrated for orthopedic procedures where a fat graft is used. We authored a research paper on a dose-response relationship in knee arthritis, specific to our bone marrow stem cell procedure, but no such data exists for orthopedic fat graft treatments.
- Stem cell therapy provides many options for the patient…to [accelerate] healing after surgical repair… Hmmm… There is no stem cell therapy being offered at this clinic, simply a fat graft procedure. While there is one study showing that injecting bone marrow concentrate into healing rotator cuff tendons can reduce postsurgical failure, there is ZERO published evidence that injecting a fat graft into a postsurgical tendon will facilitate healing.
I could go on, but suffice it to say that given that we have no evidence that a fat graft is a stem cell procedure and ZERO published clinical data that Lipogems will help the conditions being advertised, like knee arthritis, rotator cuff tears, or ligament tears, there is no need to continue. Will this data be produced? It’s likely that we’ll see some Lipogems clinical data be published. In fact, there’s a small case series in shoulder injection alone (without surgery) for rotator cuff tears that’s being presented this fall. However, in 2017, the statements on the website can’t be supported.
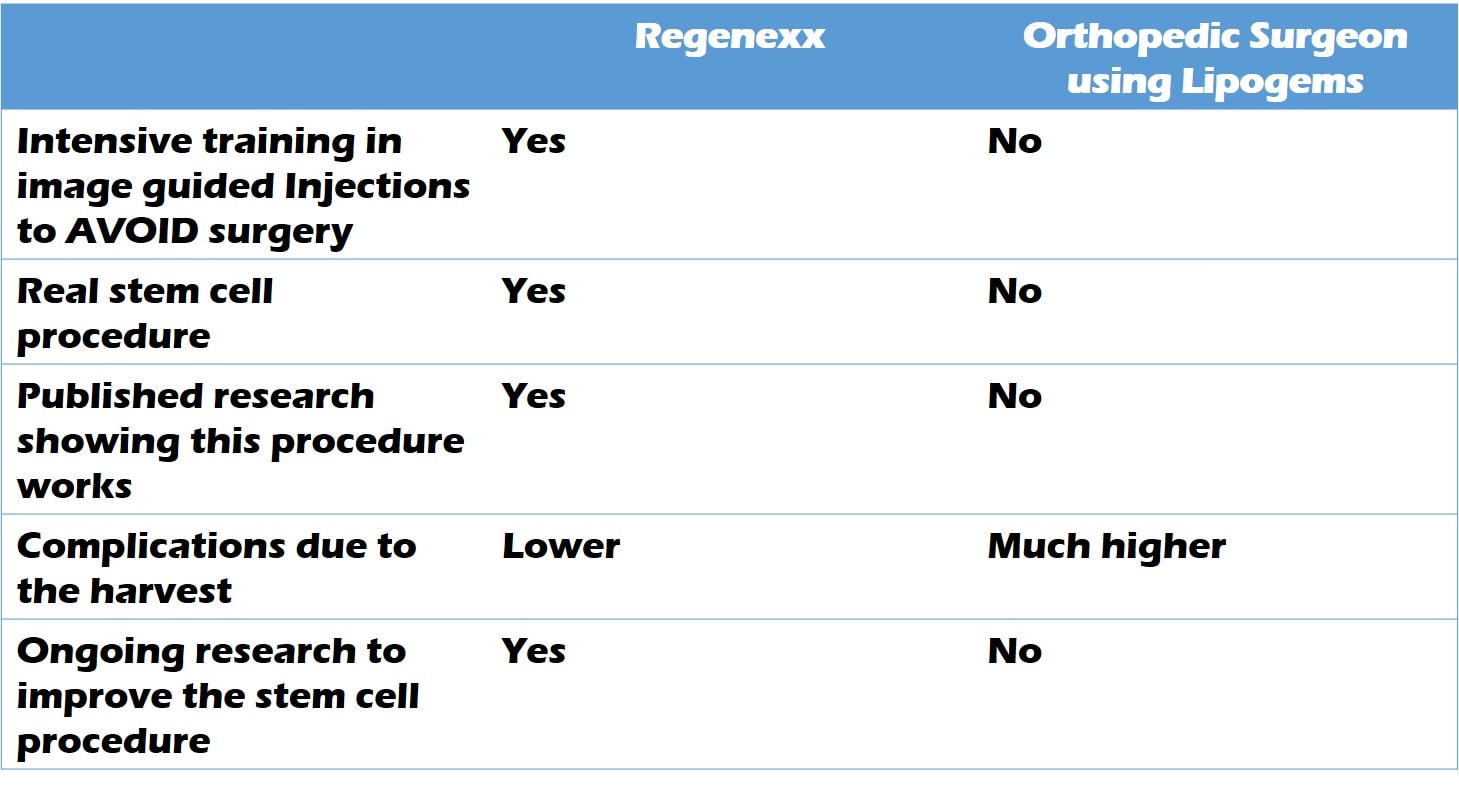
To summarize the differences between an orthopedic surgeon using Lipogems and Regenexx, I put together this little table:
The upshot? While the company that makes the Lipogems fat graft kit is very careful to tell physicians not to falsely advertise this way, this doesn’t seem to be stopping surgeons. Hence, it will be interesting to see if the company will step to the plate and stop selling to orthopedists who are advertising this procedure as something it’s not. While the company would lose sales in the short run, it would also reduce its risk of the FDA pulling the 510K-approved device and forcing it to go through a more rigorous IDE approval lasting years and requiring clinical trials. So short-term lowered sales would be better than a regulatory disaster.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.