Why the Orthopedic Research Is So Screwed Up: We Need a Neuromechanochemical Model of Pain
You’ve likely seen me complaining about the biopsychosocial (bPS) model of pain and a new concept called pain neuroscience education (PNE). This is a resurgence of the concept that disability is mostly a learned societal response to pain. PNE is mostly based on the idea that structural imaging, like MRI, has been so poor at predicting who should be in pain and who should be pain-free, therefore, pain is just hyperactive nerves plus a belief system. While it’s true that the medical literature is rife with hundreds of studies that explain why simple MRI images are so bad at diagnosing pain, this doesn’t mean that we should start blaming the pain on a nerve and a psychological boogeyman. Instead, it means that we need a new model for conceptualizing pain. Let me explain.
What Are We Missing?
When MRIs first came on the scene in the ’80s and ’90s, they were a miracle in that we could see inside the body with details in the soft tissue (i.e., more than bone) for the first time. They put the level of detail that CT scans could accomplish to shame. Hence, everyone believed that this was the ultimate diagnostic tool. However, the research tying structure to function has been fairly weak, meaning that trying to predict who hurts merely by looking at a picture of how the bones, muscles, nerves, and other tissues are arranged is sometimes no better than chance. Why? Because we now know we’re missing parts of a bigger picture, a nuance in understanding that the biopsychosocialists miss.
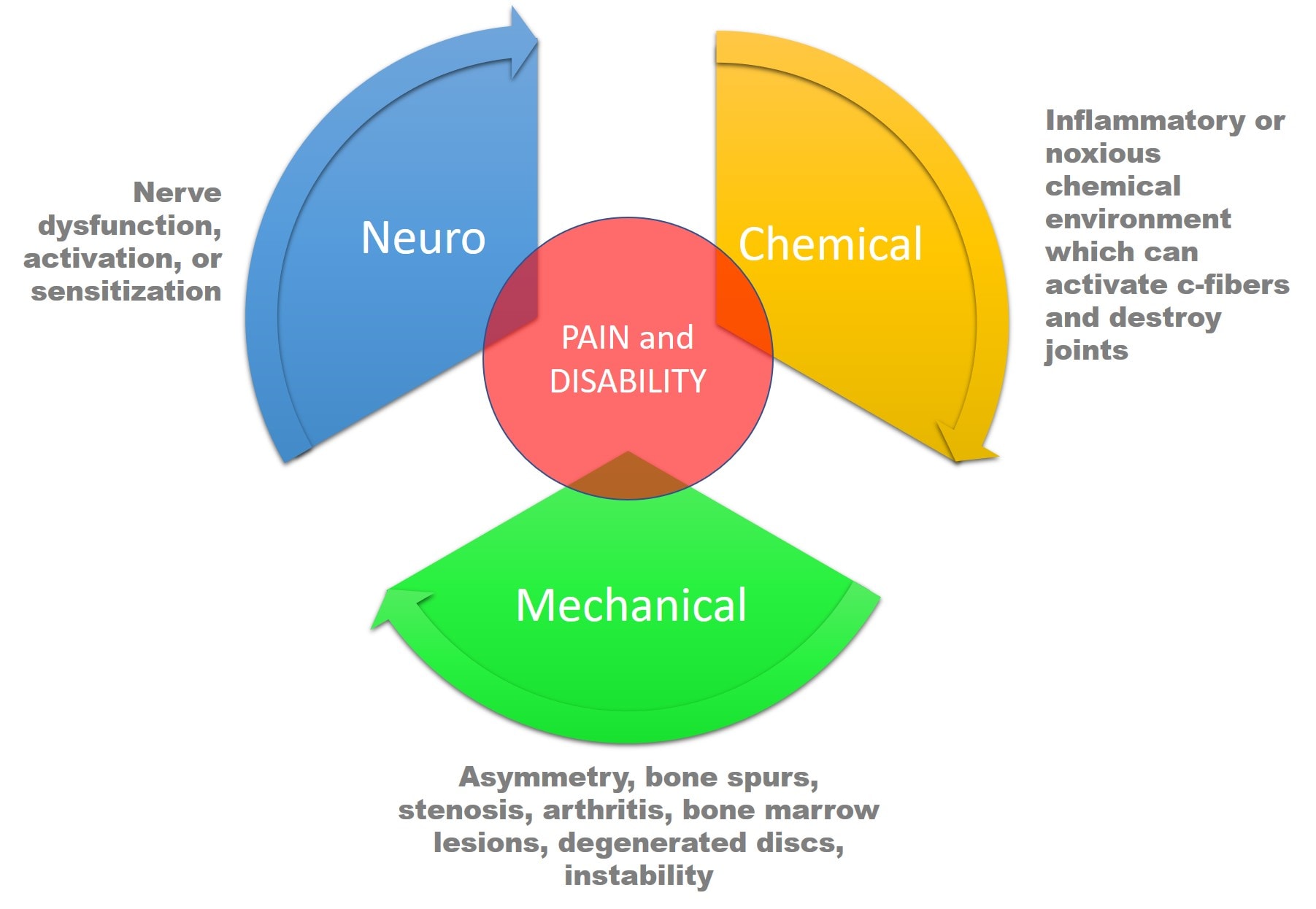
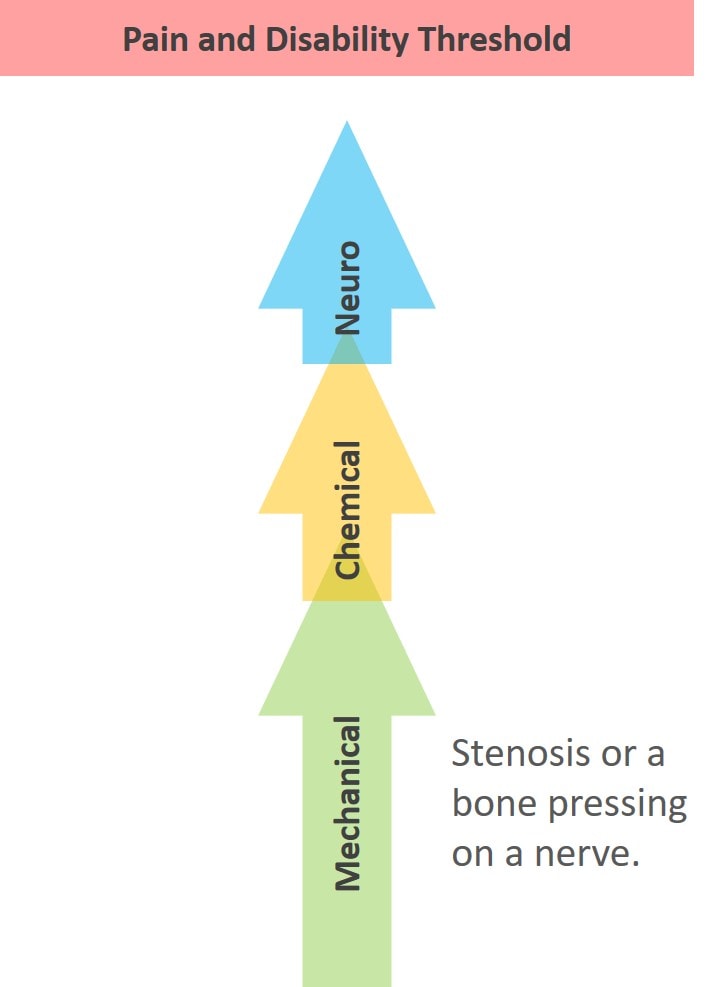
What are we missing? To keep this discussion more or less simple, we’ll just focus on the spine. There are three things that alone or in combination can cause spinal pain or disability:
- Nerve issues (neuro)
- Structural problems (mechano)
- Chemical environment (chemical)
Take a look at the diagram above. The issue is that all three of these interact in complex ways, and any of the three by itself can cause pain. Nerves (neuro) can get tweaked and lead to the release of inflammatory cytokines (chemical) and also shut down stabilizing muscles (mechanical). Or instability (mechanical) can cause a spinal nerve to get tweaked, which then causes inflammatory chemicals to be released.
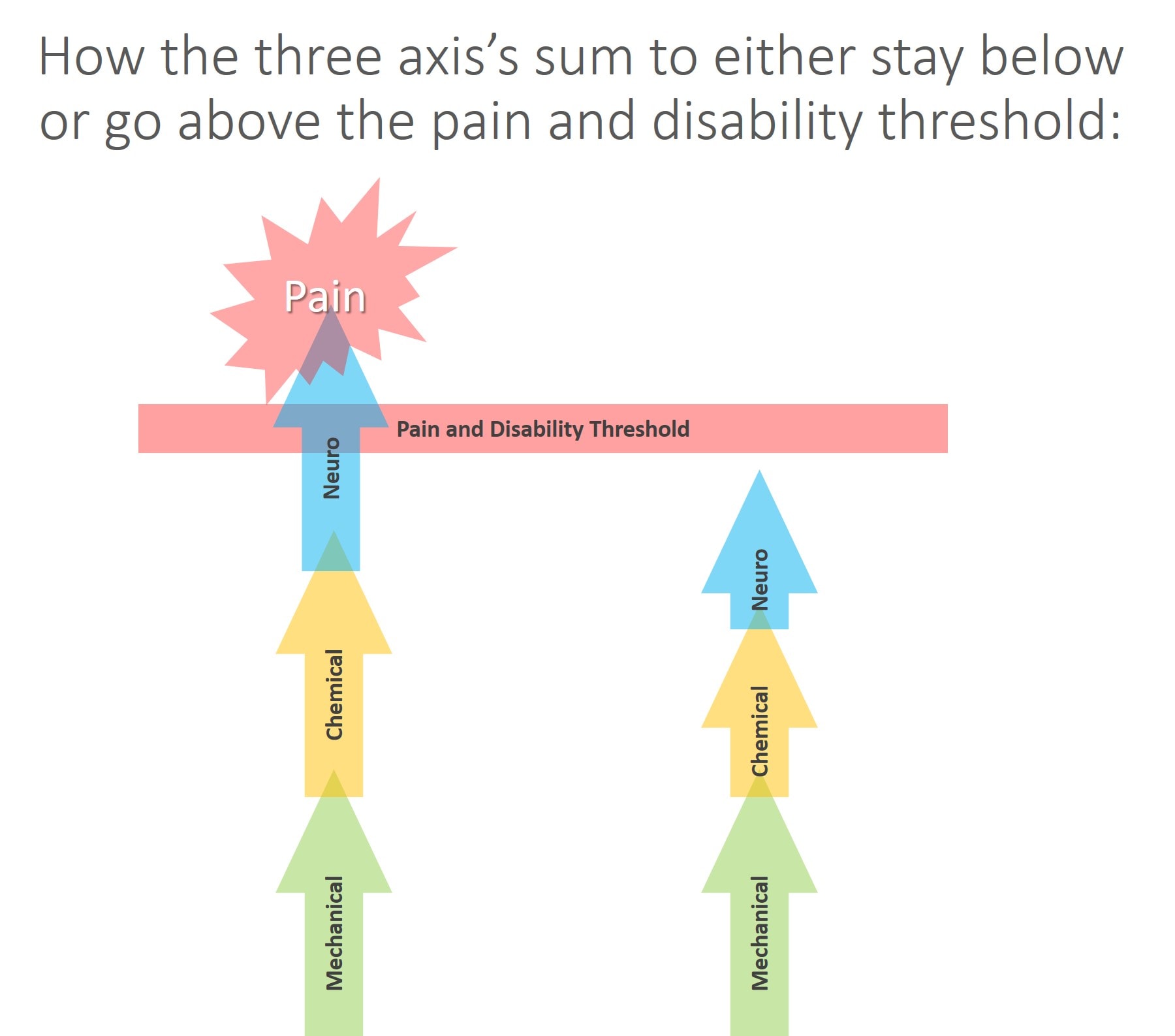
As in the diagram above, the three axes also summate, meaning that each one can add toward creating pain and disability. That means that the sum of all three can live in a subthreshold state where neuro+mechanical+chemical doesn’t trigger a pain response (on the right above). In addition, any or all of the three axes can go beyond the threshold to cause pain and disability (on the left above).
Some Examples of the Interaction Model
I’ll review two examples here, one acute and one chronic. Basically, the focus here is how one part of the model interacts with the others, which ultimately decides how each axis is ramped up and whether the sum of all the parts is below or above the pain threshold. Let me explain.
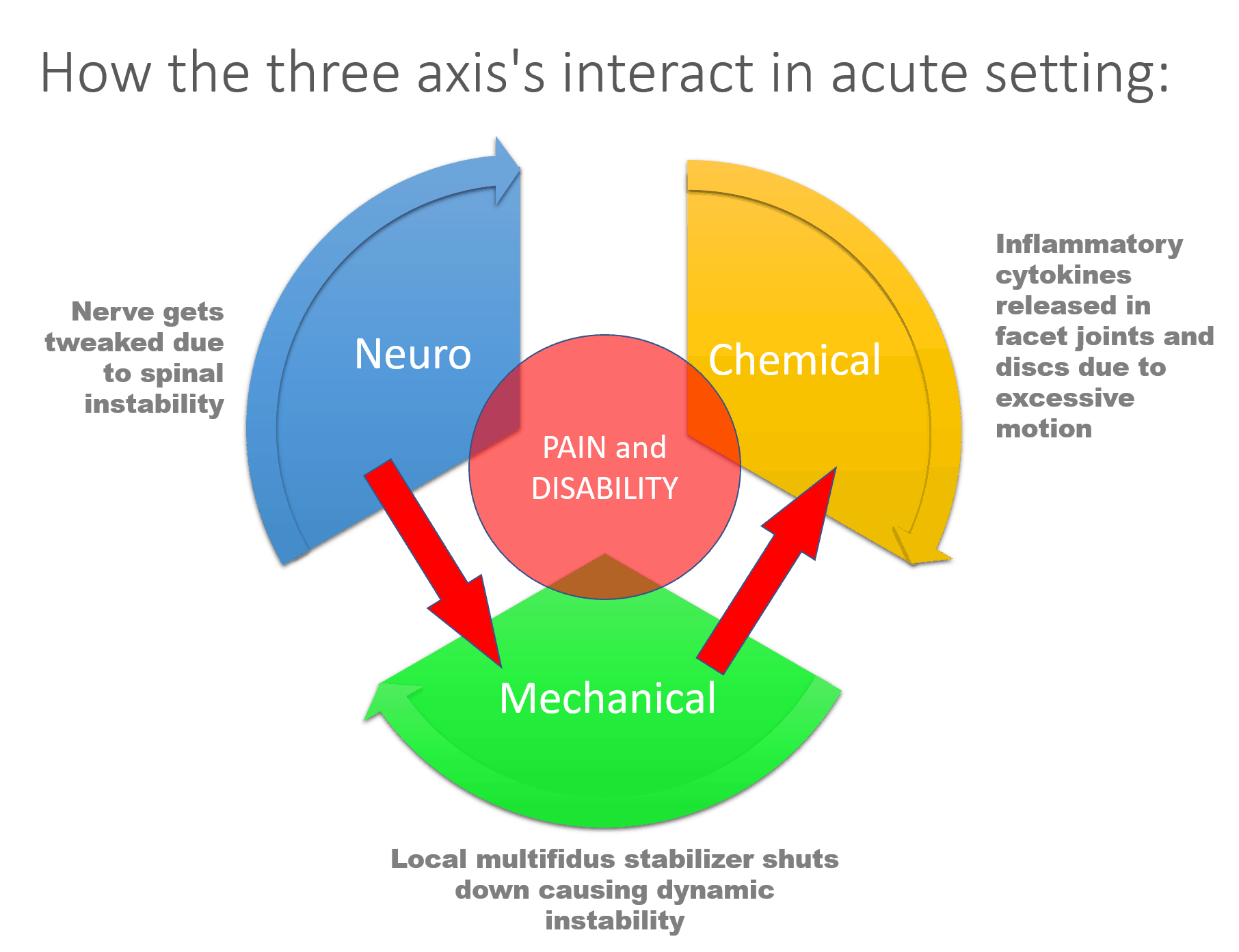
In the first example (which happened to me this morning), a spinal nerve (neuro) is tweaked due to instability (mechanical), which leads to shutdown of the local stabilizing muscles (multifidus), which causes more instability. This leads to the nerve irritation and excessive motion at the spinal level to cause inflammatory chemicals to be released from the nerve and in the local joints and disc. One part of the model interacts with all others.
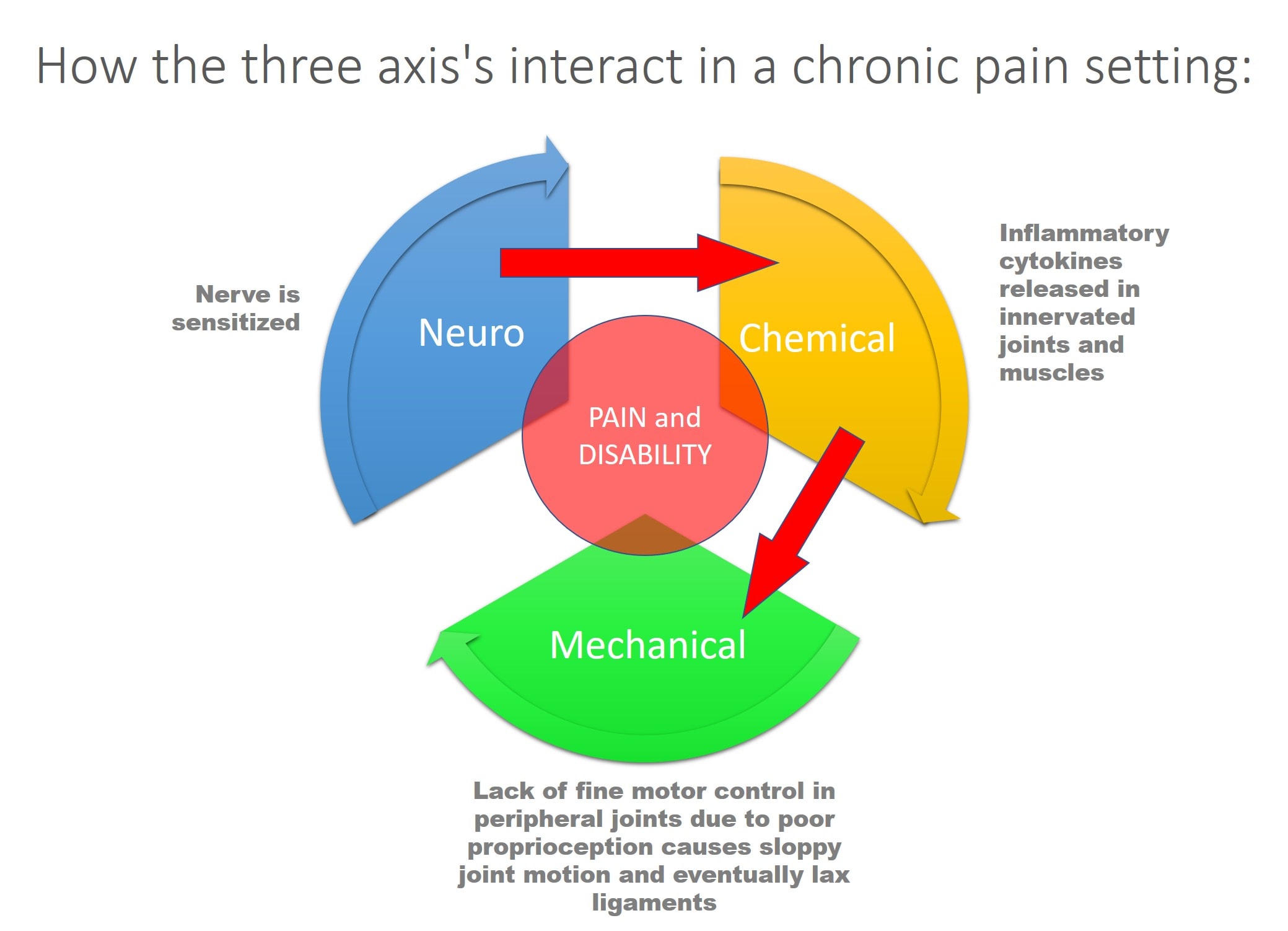
In the second example of a patient in chronic pain, a nerve is chronically sensitized. This leads to inflammatory cytokines being released in the innervated joints and muscles. This can cause a slow breakdown in those structures. In addition, the lack of normal proprioceptive (position sense) control over the innervated joint and associated muscles cause the peripheral joint (let’s say the knee) to experience excessive forces leading to degenerative instability.
Some Examples of the Summation Model
Take a look at the diagram above. Here we have a nerve that looks like it’s compressed on MRI, but it is still functioning fine and there is no local chemical inflammation trigger as the patient has low systemic inflammation. Here the structure looks like it has a problem (a bone pressing on a nerve), but the neuro part is able to compensate and the chemical piece is negligible, so no problem. The sum of all of these issues is below the pain and disability threshold. These are the patients who screw up the results of studies trying to use MRI to predict who is in pain. They look on the imaging like they should have a pain problem, but they don’t.
Now, look at the diagram above. Take that same nerve that’s being compressed and operating fine and place it in a body with high levels of systemic inflammation or a local area with same and it starts to lose function. Or let’s look at it the opposite way. Let’s say the structure looks fine, but the nerve is clinically symptomatic, which could be caused again by toxic chemical leaking from a disc (a chemical radiculitis). As you can see, in this model, the emergence of symptoms depends on how these three axes interact and summate. This would explain why some patients who have awful-looking structure have good function and why some with great looking structure have poor function.
The Evidence for the NMC Model
Neuro
A nerve is an interesting structure that extends from the spinal cord to the area it innervates. So, for example, this might be from the spinal cord to the big toe. We certainly know that nerves are involved in pain generation. More recently, much has been published on the idea of neuropathic pain and central sensitization, or nerves that are ramped up. This means that the nerves become too good at carrying pain signals and stay that way. Perhaps one of the most interesting recent developments has come in an understanding of how altered nerves can cause or facilitate mechanical/structural conditions, like osteoarthritis.
Mechano
The idea that pressure on nerves or the spinal cord can cause pain and functional problems, like weakness and lack of coordination, isn’t new nor does it need much referencing. What’s interesting is that we also know that in some instances, MRIs that show pressure on nerves aren’t great at diagnosing who should be in pain and who should be pain-free. Other studies that have tried to predict the degree of pain and disability based on nerve compression on MRI have also been disappointing. On the other hand, studies that have focused on more specific things, like the shape of the spinal canal, have been more successful. So while looking at structure alone can be helpful if it fits with the patient exam (i.e., a midline herniated disc at L5–S1 placing pressure on the descending S1 nerve), by itself, it’s not all that helpful in predicting poor function or pain.
We also know that structure itself may or may not be accurate in predicting disability. As an example, while changes in cartilage in the knee or tears in the meniscus may not be associated with pain, we do know that changes in the bone called bone marrow lesions are more likely to be painful. Or take, for example, the research showing that while certain bone spurs in the hip are protective of the joint, other hip-joint shapes are associated with the development of osteoarthritis. Hence, in conclusion, structure by itself may or may not be painful, but it’s one part of a bigger picture of why patients hurt.
Chemical
The idea that the chemical composition of an area impacts pain has blossomed over the last decade as our ability to cheaply assay various inflammatory cytokines has increased. Take, for example, research showing that rotator cuff tear pain was more associated with cytokine levels than whether or not the tear healed on post-op MRI. Or another study showing that pain and disability after a herniated disc were directly correlated with serum inflammatory levels, meaning more whole-body inflammation equaled longer recovery.
The upshot? Pain and disability turn out to be a complex interplay of nerve activation, structural, and chemical factors. This is why our research on using simple MRI techniques to predict pain has been so “all over the map.” This is also why the results of randomized controlled trials in orthopedic surgery studies have been so disappointing. These surgeries were only designed to address one axis of the three that control whether someone has pain. I hope that this concept of an NMC model helps to also stem the tide of physical therapists who believe that talking patients out of their irritated nerves is an effective therapy. Instead, we need to be focusing on ways to measure the nerve activation, structural, and chemical status of every body area to more effectively diagnose and treat what’s wrong.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.