Stem Cell Therapy Seminar King Still Hasn’t Cleaned Up Its Act
I have been blogging about stem cell therapy seminar king, Stem Cell Institute of America, and its issues for a while. The company has been offering dead amniotic “stem cell” injections at chiropractic offices and pawning them off as live stem cell procedures for years. These past few months, it appeared to try to come clean by sending out a strongly worded e-mail to its providers. However, local news ads in the Los Angeles area show the company still has compliance problems.
What Is Stem Cell Institute of America?
Stem Cell Institute of America (SCIA) is a chiropractic-practice management group that focuses on increasing revenue for its client chiro offices. One of the ways they do that is to offer new services. In this case, they most often suggest hiring a nurse to perform injections of amniotic tissue, and the company has traditionally called these stem cell procedures. They then aggressively advertise “stem cell” therapy seminars, often showing dramatic before and after X-rays at the event while suggesting to the elderly that they can regrow them significant new cartilage in “bone on bone” knees.
Is any of this true? Nope.
First, what’s being injected is regulated by the FDA to be a dead cell product. In addition, no credible scientific data exists that shows that this stuff has any live and functional stem cells. See my two videos below to dive deeper into those topics:
Next, X-rays are not an accurate way to measure cartilage regrowth and are easily susceptible to manipulation, as I demonstrated in this image:
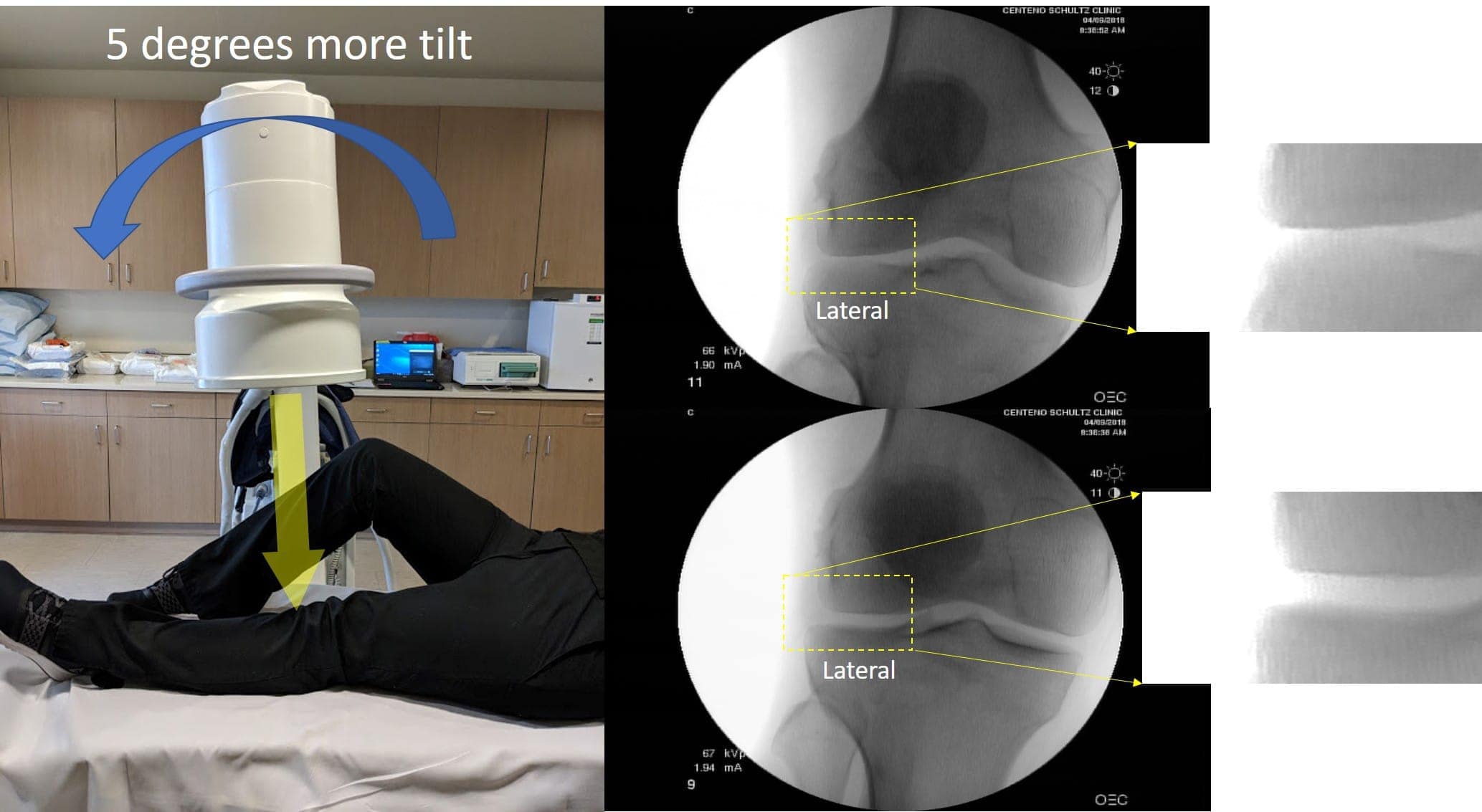
Here I slightly changed the angle of the X-ray beam and dramatically increased the width of my knee joint. For more details, see the link above. In addition, no real stem cell therapy will actually regrow significant new cartilage in severely arthritic knees (despite helping pain and function).
Then there are the stem cell therapy seminars themselves. The video below is a look inside what’s on the slides the company was presenting versus reality. In addition, it goes over the fact that the ex-company president is a convicted felon:
Did SCIA Try to Clean Up Its Stem Cell Therapy Seminar?
SCIA sent out a very direct and strongly worded message to its chiropractic clients a few months back. In it, they stated that these sites couldn’t advertise “stem cell therapy” if they were using amniotic or cord birth tissues. That made sense, as I’ve been saying for years that the amniotic and umbilical cord therapies the company had advertised as “stem cell therapy” didn’t actually have live and functional stem cells.
When this announcement was made, I had my doubts that the company could make this switch, as this is not a provider network but a loose collection of chiropractic offices that pay SCIA for marketing resources, services, and products. In fact, I postulated that SCIA would need a name change, as most of their sites do not use bone marrow. In fact, at their annual conference this year, the providers in attendance reported that 81% of them used amniotic or umbilical cord tissues and only a minority was capable of using bone marrow (a real stem cell procedure). In fact, the most recent iteration of their provider contract that I was able to review and that was being sent by SCIA to its providers had this paragraph:
““No warranties, promises or representations of any kind, expressed or implied, are given as to the nature, content, effectiveness, legality or safety of any product represented in the presentation, including the presence or population of viable mesenchymal stem cells.“
Hence, even SCIA admits it has issues with the idea that it provides “stem cell therapy.” So much so that they are unwilling to warranty that the products represented in their presentations have stem cells. In addition, looking at all of the evidence, IMHO the only way that SCIA could remain in compliance itself would be to drop the phrase “stem cell” from its name and stop advertising “stem cell” seminars. They seem to have taken a half step in this direction by placing this on their main website:
“Looking for a reliable, caring doctor to provide you with our cutting-edge stem cell treatments Or advanced regenerative treatment options?”
Notice the poor syntax with the capitalized “Or” looks like that part was added. To make sure, I checked the Internet Archive for December 2017, and this is what I found:
“Looking for a reliable, caring doctor to provide you with our cutting-edge stem cell treatments?”
Looks like I was right, they simply added: “Or advanced regenerative treatment options.”
SCIA TV Ads
Recently, a medical provider in California sent me a screenshot of a local SCIA ad:
Huh? The new language isn’t here. In fact, you would be led to believe that if you call this number, you can attend a seminar where you will hear only about “stem cell therapy.”
There are only two conclusions you can draw from this commercial. Either every provider in this area is not performing only amniotic or cord dead tissue therapy or SCIA is back to its old tricks again. Saying one thing, but doing another. I guess another option is that given the structure of the organization, it’s unclear whether this ad was even placed by SCIA or a local chiro group that is using the company’s materials.
The upshot? As I often tell my colleagues, you just can’t make this stuff up. SCIA made a big deal a few months back about cleaning up its act and even made some minor changes to its website. However, this most recent TV ad isn’t compliant with what SCIA says it must now do to stay compliant with FDA regulations. So what gives?

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.